|
Four-center Two-electron Bond
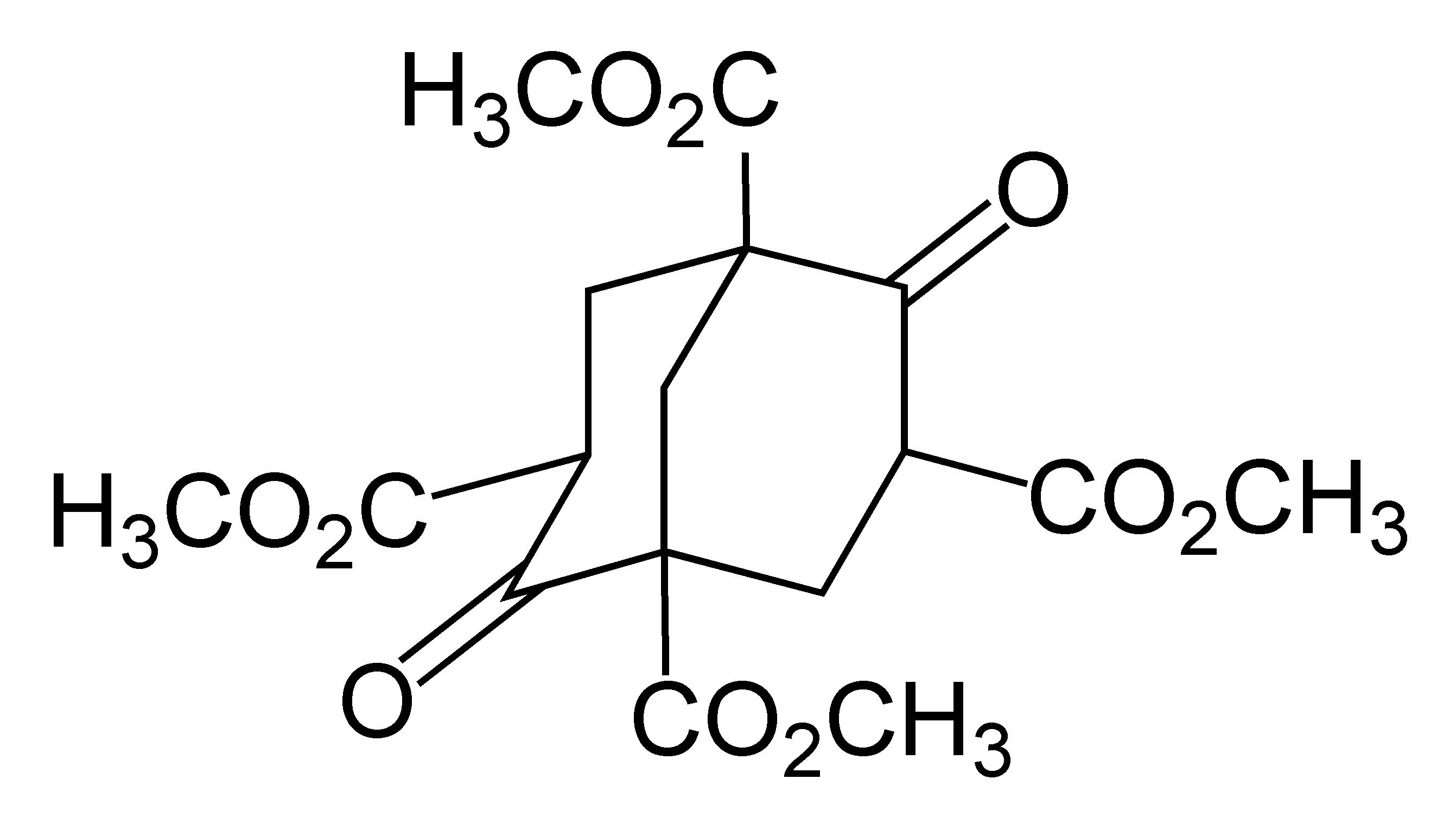
A 4-center 2-electron (4c–2e) bond is a type of chemical bond in which four atoms share two electrons in bonding, with a net bond order of . This type of bonding differs from the usual covalent bond, which involves two atoms sharing two electrons (2c–2e bonding). Four-center two-electron bonding is postulated in certain cluster compounds. For instance, the borane anion, is a octahedron with an additional proton attached to one of the triangular faces. As a result, the octahedron is distorted and a B–B–B–H rhomboid ring can be identified in which this 4c–2e bonding takes place. This type of bonding is associated with electron deficient rhomboid rings in general and is a relatively new research field, fitting in with the already well established three-center two-electron bond. An example of a purely organic compound with four-center two-electron bonding is the adamantyl dication. The bond joins the four bridgehead atoms in a tetrahedral geometry. Tetracyanoethylene f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force and hydrogen bonding. Strong chemical bonding arises from the sharing or transfer of electrons between the participating atoms. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Three-center Two-electron Bond
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one ''non''-bonding, and one ''anti''-bonding. The two electrons go into the bonding orbital, resulting in a net bonding effect and constituting a chemical bond among all three atoms. In many common bonds of this type, the bonding orbital is shifted towards two of the three atoms instead of being spread equally among all three. Example molecules with 3c–2e bonds are the trihydrogen cation () and diborane (). In these two structures, the three atoms in each 3c-2e bond form an angular geometry, leading to a bent bond. Boranes and carboranes An extended version of the 3c–2e bond model features heavily in cluster compounds described by the polyhedral skeletal electron pair theory, such as boranes and carboranes. These molecules derive their stability from having a com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () (''di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetracyanoethylene
Tetracyanoethylene (TCNE) is organic compound with the formula . It is a colorless solid, although samples are often off-white. It is an important member of the cyanocarbons. Synthesis and reactions TCNE is prepared by brominating malononitrile in the presence of potassium bromide to give the KBr-complex, and dehalogenating with copper. Oxidation of TCNE with hydrogen peroxide gives the corresponding epoxide, which has unusual properties. In the presence of base, TCNE reacts with malononitrile to give salts of pentacyanopropenide: :C2(CN)2 + CH2(CN)2 -> NC)2C-C(CN)-C(CN)2 + CN- + 2H+ Redox chemistry TCNE is an electron acceptor. Cyano groups have low energy π* orbitals, and the presence of four such groups, with their π systems (conjugated) to the central double bond, gives rise to an electrophilic alkene. TCNE is reduced at -0.27 V vs ferrocene/ ferrocenium: :C2(CN)4 + e- -> 2(CN)4 Because of its ability to accept an electron, TCNE has been used to prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TCNE Dianion Delocalization
Tetracyanoethylene (TCNE) is organic compound with the formula . It is a colorless solid, although samples are often off-white. It is an important member of the cyanocarbons. Synthesis and reactions TCNE is prepared by brominating malononitrile in the presence of potassium bromide to give the KBr-complex, and dehalogenating with copper. Oxidation of TCNE with hydrogen peroxide gives the corresponding epoxide, which has unusual properties. In the presence of base, TCNE reacts with malononitrile to give salts of pentacyanopropenide: :C2(CN)2 + CH2(CN)2 -> NC)2C-C(CN)-C(CN)2 + CN- + 2H+ Redox chemistry TCNE is an electron acceptor. Cyano groups have low energy π* orbitals, and the presence of four such groups, with their π systems (conjugated) to the central double bond, gives rise to an electrophilic alkene. TCNE is reduced at -0.27 V vs ferrocene/ferrocenium: :C2(CN)4 + e- -> 2(CN)4 Because of its ability to accept an electron, TCNE has been used to prepare numerous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge length of the cube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adamantyl
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name ''adamantane'', which is derived from the Greek ''adamantinos'' (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid. The discovery of adamantane in petroleum in 1933 launched a new field of chemistry dedicated to the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials, and thermally stable lubricants. History and synthesis In 1924, H. Decker suggested the existence of adamantane, which he called decaterpene. The first attempted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhomboid
Traditionally, in two-dimensional geometry, a rhomboid is a parallelogram in which adjacent sides are of unequal lengths and angles are non-right angled. A parallelogram with sides of equal length ( equilateral) is a rhombus but not a rhomboid. A parallelogram with right angled corners is a rectangle but not a rhomboid. The term ''rhomboid'' is now more often used for a rhombohedron or a more general parallelepiped, a solid figure with six faces in which each face is a parallelogram and pairs of opposite faces lie in parallel planes. Some crystals are formed in three-dimensional rhomboids. This solid is also sometimes called a rhombic prism. The term occurs frequently in science terminology referring to both its two- and three-dimensional meaning. History Euclid introduced the term in his ''Elements'' in Book I, Definition 22, Euclid never used the definition of rhomboid again and introduced the word parallelogram in Proposition 34 of Book I; ''"In parallelogrammic areas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem
Chem may refer to: * Chemistry practical waali mam * Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" *Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel * CHEM-DT, a Canadian television channel See also *Chemo (other) Chemo is a prefix meaning ''chemical'' and commonly used as an abbreviation for chemotherapy. Chemo may also refer to: People * Chemo (musician), an English musician now known as Forest DLG * Chemo Soto, a Puerto Rican politician * José del Sol ... * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
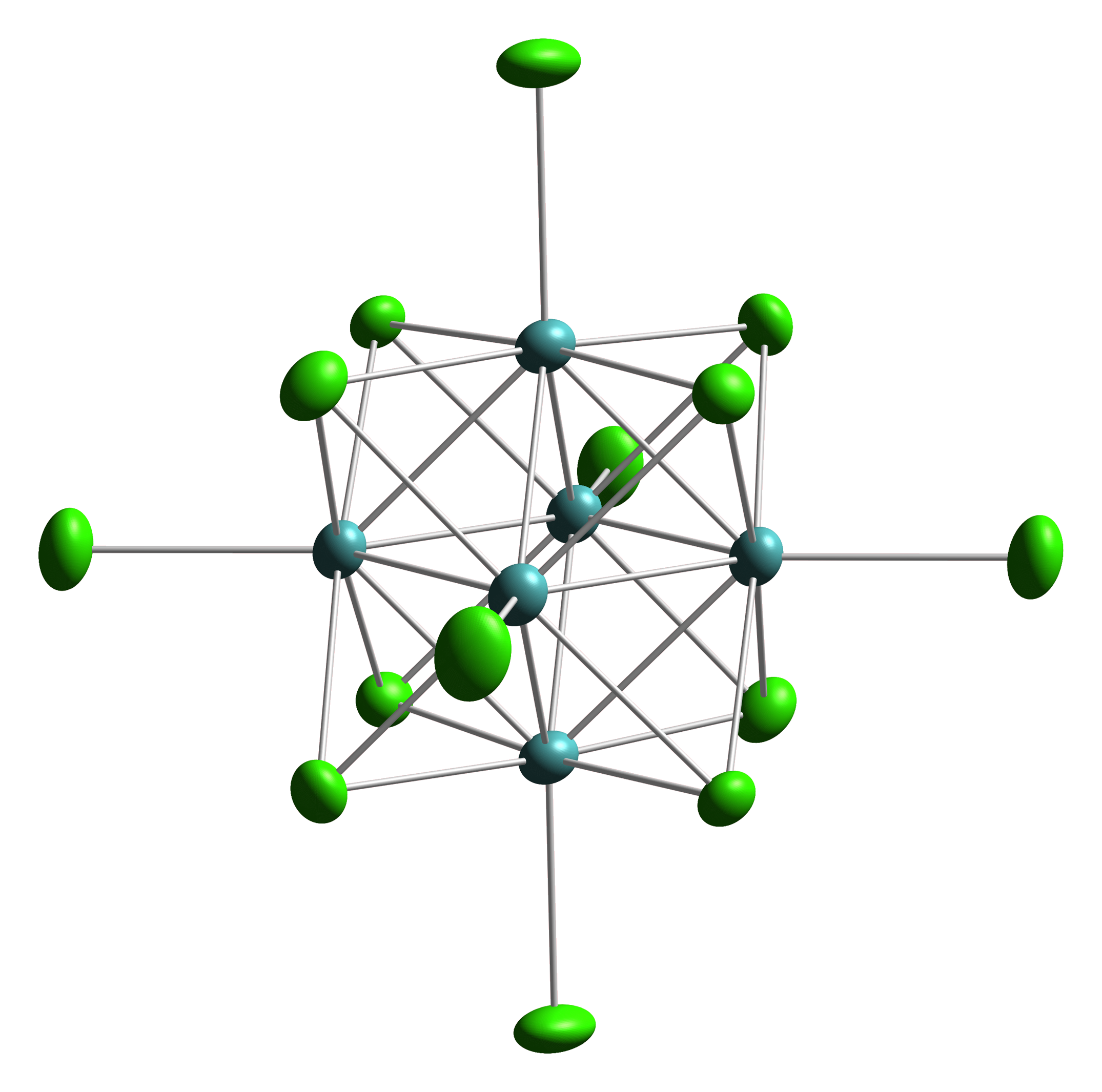
Octahedral Cluster
Octahedral clusters are inorganic or organometallic cluster compounds composed of six metals in an octahedral array.Eric J. Welch and Jeffrey R. Long ''Atomlike Building Units of Adjustable Character: Solid-State and Solution Routes to Manipulating Hexanuclear Transition Metal Chalcohalide Clusters'' in Progress in Inorganic Chemistry, Volume 54 Kenneth D. Karlin 2005''Link/ref> Many types of compounds are known, but all are synthetic. Octahedral chalcogenide and halide clusters These compounds are bound together by metal-metal bonding as well as two kinds of ligands. Ligands that span the faces or edges of the M6 core are labeled Li, for ''inner'' (innen in the original German description), and those ligands attached only to one metal are labeled outer, or La for ''ausser''. Typically, the outer ligands can be exchanged whereas the bridging ligands are more inert toward substitution. Face-capped halide clusters The premier example is of the class is Mo6Cl142−. This dianion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |