|
Fluorescence Detection
A fluorometer, fluorimeter or fluormeter is a device used to measure parameters of visible spectrum fluorescence: its intensity and wavelength distribution of emission spectrum after excitation by a certain spectrum of light. These parameters are used to identify the presence and the amount of specific molecules in a medium. Modern fluorometers are capable of detecting fluorescent molecule concentrations as low as 1 part per trillion. Fluorescence analysis can be orders of magnitude more sensitive than other techniques. Applications include chemistry/biochemistry, medicine, environmental monitoring. For instance, they are used to measure chlorophyll fluorescence to investigate plant physiology. Components and design Typically fluorometers utilize a double beam. These two beams work in tandem to decrease the noise created from radiant power fluctuations. The upper beam is passed through a filter or monochromator and passes through the sample. The lower beam is passed through an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Stray Light
Stray light is light in an optical system which was not intended in the design. The light may be from the intended source, but follow paths other than intended, or it may be from a source other than that intended. This light will often set a working limit on the dynamic range of the system; it limits the signal-to-noise ratio or contrast ratio, by limiting how dark the system can be. Ocular straylight is stray light in the human eye. Optical systems Monochromatic light Optical measuring instruments that work with monochromatic light, such as spectrophotometers, define stray light as light in the system at wavelengths (colors) other than the one intended. The stray light level is one of the most critical specifications of an instrument. For instance, intense, narrow absorption bands can easily appear to have a peak absorption less than the true absorption of the sample because the ability of the instrument to measure light transmission through the sample is limited by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
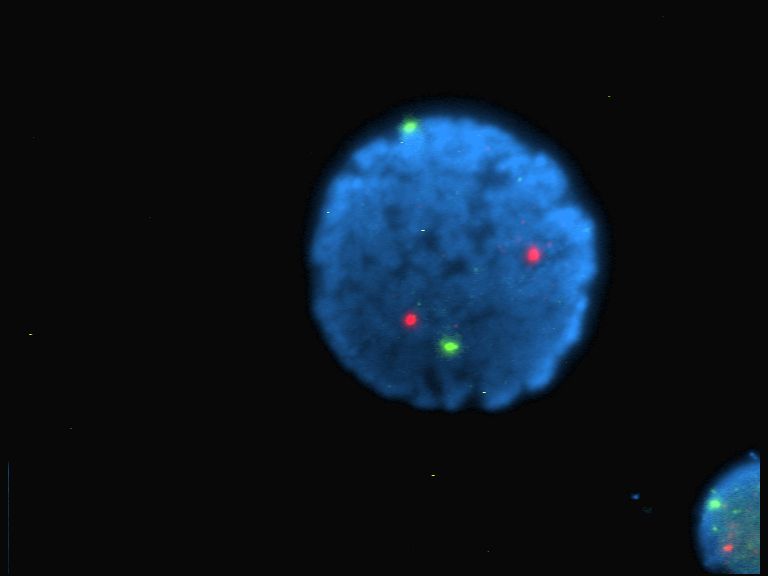
Histology
Histology, also known as microscopic anatomy or microanatomy, is the branch of biology that studies the microscopic anatomy of biological tissue (biology), tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into ''organology'', the study of organs, ''histology'', the study of tissues, and ''cytology'', the study of cell (biology), cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms. Biological tissues Animal tissue classification There are four basic types of animal tissues: muscle tissue, nervous tissue, connective tissue, and epithelial tissue. All animal tissues are considered to be subtypes of these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Thioflavin
Thioflavins are fluorescent dyes that are available as at least two compounds, namely Thioflavin T and Thioflavin S. Both are used for histology staining and biophysical studies of protein aggregation. In particular, these dyes have been used since 1989 to investigate amyloid formation. They are also used in biophysical studies of the electrophysiology of bacteria. Thioflavins are corrosive, irritant, and acutely toxic, causing serious eye damage. Thioflavin T has been used in research into Alzheimer's disease and other neurodegenerative diseases. Thioflavin T Thioflavin T (Basic Yellow 1, Methylene yellow, CI 49005, or ThT) is a benzothiazole salt obtained by the methylation of dehydrothiotoluidine with methanol in the presence of hydrochloric acid. The dye is widely used to visualize and quantify the presence of misfolded protein aggregates called amyloid, both ''in vitro'' and ''in vivo'' (e.g., plaques composed of amyloid beta found in the brains of Alzheimer's disease pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Denaturation (biochemistry)
In biochemistry, denaturation is a process in which proteins or nucleic acids lose folded structure present in their native state due to various factors, including application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent (e.g., alcohol or chloroform), agitation, radiation, or heat. If proteins in a living cell are denatured, this results in disruption of cell activity and possibly cell death. Protein denaturation is also a consequence of cell death. Denatured proteins can exhibit a wide range of characteristics, from conformational change and loss of solubility or dissociation of cofactors to aggregation due to the exposure of hydrophobic groups. The loss of solubility as a result of denaturation is called ''coagulation''. Denatured proteins, e.g., metalloenzymes, lose their 3D structure or metal cofactor, and therefore, cannot function. Proper protein folding is key to whether a globular or memb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Alkaline Phosphatase
The enzyme alkaline phosphatase (ALP, alkaline phenyl phosphatase, also abbreviated PhoA) is a phosphatase with the physiological role of dephosphorylating compounds. The enzyme is found across a multitude of organisms, prokaryotes and eukaryotes alike, with the same general function, but in different structural forms suitable to the environment they function in. Alkaline phosphatase is found in the periplasmic space of '' E. coli'' bacteria. This enzyme is heat stable and has its maximum activity at high pH. In humans, it is found in many forms depending on its origin within the body – it plays an integral role in metabolism within the liver and development within the skeleton. Due to its widespread prevalence in these areas, its concentration in the bloodstream is used by diagnosticians as a biomarker in helping determine diagnoses such as hepatitis or osteomalacia. The level of alkaline phosphatase in the blood is checked through the ALP test, which is often par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to macromolecules, serving as a markers (or dyes, or tags, or reporters) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, such as fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Pasteurization
In food processing, pasteurization (American and British English spelling differences#-ise, -ize (-isation, -ization), also pasteurisation) is a process of food preservation in which packaged foods (e.g., milk and fruit juices) are treated with mild heat, usually to less than , to eliminate pathogens and extend shelf life. Pasteurization either destroys or deactivates microorganisms and enzymes that contribute to food spoilage or the risk of disease, including vegetative bacteria, but most Endospore, bacterial spores survive the process. Pasteurization is named after the French microbiologist Louis Pasteur, whose research in the 1860s demonstrated that thermal processing would deactivate unwanted microorganisms in wine. Spoilage enzymes are also inactivated during pasteurization. Today, pasteurization is used widely in the dairy industry and other food processing industries for food preservation and food safety. By the year 1999, most liquid products were heat treated in a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows Tetrahedral molecular geometry, tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Chemiluminescence
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction, i.e. a chemical reaction results in a flash or glow of light. A standard example of chemiluminescence in the laboratory setting is the luminol test. Here, blood is indicated by luminescence upon contact with iron in hemoglobin. When chemiluminescence takes place in living organisms, the phenomenon is called bioluminescence. A light stick emits light by chemiluminescence. Physical description As in many chemical reactions, chemiluminescence starts with the combining of two compounds, say A and B, to give a product C. Unlike most chemical reactions, the product C converts to a further product, which is produced in an electronically excited state often indicated with an asterisk: : A + B → C : C → D* D* then emits a photon (''h''ν), to give the ground state of D: I : D* → D + ''h''ν In theory, one photon of light should be given off for each mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |