|
Ferredoxins
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium '' Clostridium pasteurianum''. Another redox protein, isolated from spinach chloroplasts, was termed "chloroplast ferredoxin". The chloroplast ferredoxin is involved in both cyclic and non-cyclic photophosphorylation reactions of photosynthesis. In non-cyclic photophosphorylation, ferredoxin is the last electron acceptor thus reducing the enzyme NADP+ reductase. It accepts electrons produced from sunlight- excited chlorophyll and transfers them to the enzyme ferredoxin: NADP+ oxidoreductase . Ferredoxins are small proteins containing iron and sulfur atoms organized as iron–sulfur clusters. These biological "capacitors" can acc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron–sulfur Protein
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, formin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
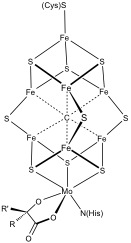
Iron–sulfur Cluster
Iron–sulfur clusters are molecular ensembles of iron and sulfide. They are most often discussed in the context of the biological role for iron–sulfur proteins, which are pervasive. Many Fe–S clusters are known in the area of organometallic chemistry and as precursors to synthetic analogues of the biological clusters. It is supposed that the last universal common ancestor had many iron-sulfur clusters. In biology Iron–sulfur clusters occur in many biological systems, often as components of electron transfer proteins. The ferredoxin proteins are the most common Fe–S proteins in nature. They feature either 2Fe–2S or 4Fe–4S centers. They occur in all branches of life. Fe–S clusters can be classified according to their Fe:S stoichiometry Fe–2S Fe–3S Fe–4S and Fe–4S The Fe–4Sclusters occur in two forms: normal ferredoxins and high potential iron proteins (HiPIP). Both adopt cuboidal structures, but they utilize different oxidation states. They are f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area around Rome, Italy. Through the expansion of the Roman Republic, it became the dominant language in the Italian Peninsula and subsequently throughout the Roman Empire. It has greatly influenced many languages, Latin influence in English, including English, having contributed List of Latin words with English derivatives, many words to the English lexicon, particularly after the Christianity in Anglo-Saxon England, Christianization of the Anglo-Saxons and the Norman Conquest. Latin Root (linguistics), roots appear frequently in the technical vocabulary used by fields such as theology, List of Latin and Greek words commonly used in systematic names, the sciences, List of medical roots, suffixes and prefixes, medicine, and List of Latin legal terms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubredoxin
Rubredoxins are a class of low-molecular-weight iron-containing proteins found in sulfur-metabolizing bacteria and archaea. Sometimes rubredoxins are classified as iron-sulfur proteins; however, in contrast to iron-sulfur proteins, rubredoxins do not contain inorganic sulfide. Like cytochromes, ferredoxins and Rieske proteins, rubredoxins are thought to participate in electron transfer in biological systems. Recent work in bacteria and algae have led to the hypothesis that some rubredoxins may instead have a role in delivering iron to metalloproteins. Structure The 3-D structures of a number of rubredoxins have been solved. The fold belongs to the α+β class, with 2 α-helices and 2-3 β-strands. Rubredoxin active site contains an iron ion which is coordinated by the sulfurs of four conserved cysteine residues forming an almost regular tetrahedron. This is sometimes denoted as a Fe-0Sor an Fe1S0 system, in analogy to the nomenclature for iron-sulfur proteins. While the vas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. It equals the interior potential minus the exterior potential. This is the energy (i.e. work) per charge which is required to move a (very small) positive charge at constant velocity across the cell membrane from the exterior to the interior. (If the charge is allowed to change velocity, the change of kinetic energy and production of radiation must be taken into account.) Typical values of membrane potential, normally given in units of milli volts and denoted as mV, range from −80 mV to −40 mV. For such typical negative membrane potentials, positive work is required to move a positive charge from the interior to the exterior. However, thermal kinetic energy allows ions to overcome the potential difference. For a selectively permeable membrane, this permits a net flow against the gradient. This is a kind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyruvate Synthase
In enzymology, a pyruvate synthase () is an enzyme that catalysis, catalyzes the interconversion of pyruvate and acetyl-CoA. It is also called pyruvate:ferredoxin oxidoreductase (PFOR). The relevant equilibrium catalysed by PFOR is: :pyruvate + CoA + oxidized ferredoxin \rightleftharpoons acetyl-CoA + CO2 + reduced ferredoxin The 3 substrate (biochemistry), substrates of this enzyme are pyruvate, coenzyme A, CoA, and oxidized ferredoxin, whereas its 3 product (chemistry), products are acetyl-CoA, carbon dioxide, CO2, and reduced ferredoxin. Function This enzyme participates in 4 metabolism, metabolic pathways: pyruvate metabolism, propanoate metabolism, butanoate metabolism, and reductive carboxylate cycle ( fixation). Its major role is the extraction of reducing equivalents by the decarboxylation. In aerobic organisms, this conversion is catalysed by pyruvate dehydrogenase, also uses thiamine pyrophosphate (TPP) but relies on lipoate as the electron acceptor. Unlike the ae ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide Dehydrogenase
In enzymology, carbon monoxide dehydrogenase (CODH) () is an enzyme that catalyzes the chemical reaction :CO + H2O + A \rightleftharpoons CO2 + AH2 The chemical process catalyzed by carbon monoxide dehydrogenase is similar to the water-gas shift reaction. The 3 substrates of this enzyme are CO, H2O, and A, whereas its two products are CO2 and AH2. A variety of electron donors/receivers (Shown as "A" and "AH2" in the reaction equation above) are observed in micro-organisms which utilize CODH. Several examples of electron transfer cofactors have been proposed, including Ferredoxin, NADP+/NADPH and flavoprotein complexes like flavin adenine dinucleotide (FAD) as well as hydrogenases. CODHs support the metabolisms of diverse prokaryotes, including methanogens, aerobic carboxidotrophs, acetogens, sulfate-reducers, and hydrogenogenic bacteria. The bidirectional reaction catalyzed by CODH plays a role in the carbon cycle allowing organisms to both make use of CO as a sour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyceraldehyde-3-phosphate Dehydrogenase (ferredoxin)
In enzymology, a glyceraldehyde-3-phosphate dehydrogenase (ferredoxin) () is an enzyme that catalyzes the chemical reaction :D-glyceraldehyde-3-phosphate + H2O + 2 oxidized ferredoxin \rightleftharpoons 3-phospho-D-glycerate + 2 H+ + 2 reduced ferredoxin The 3 substrates of this enzyme are D-glyceraldehyde-3-phosphate, H2O, and oxidized ferredoxin, whereas its 3 products are 3-phospho-D-glycerate, H+, and reduced ferredoxin. This enzyme belongs to the family of oxidoreductases, specifically those acting on the aldehyde or oxo group of donor with an iron-sulfur protein as acceptor. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivi ... of this enzyme class is D-glyceraldehyde-3-phosphate:ferredoxin oxidoreductase. Other names in common use include GAPOR, glycer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form, whereas NADP is the oxidized form. NADP is used by all forms of cellular life. NADP is essential for life because it is needed for cellular respiration. NADP differs from NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine moiety. This extra phosphate is added by NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase adding the extra phosphate group. ADP-ribosyl cyclase allows for synthesis from nicotinamide in the salvage pathway, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Table Of Standard Reduction Potentials For Half-reactions Important In Biochemistry
The values below are standard apparent reduction potentials for electro-biochemical half-reactions measured at 25 °C, 1 atmosphere and a pH of 7 in aqueous solution. The actual physiological potential depends on the ratio of the reduced () and oxidized () forms according to the Nernst equation and the thermal voltage. When an oxidizer () accepts a number ''z'' of electrons () to be converted in its reduced form (), the half-reaction is expressed as: : + ''z'' → The reaction quotient (r) is the ratio of the chemical activity (''a''i) of the reduced form (the reductant, ''a''Red) to the activity of the oxidized form (the oxidant, ''a''ox). It is equal to the ratio of their concentrations (''C''i) only if the system is sufficiently diluted and the activity coefficients (''γ''i) are close to unity (''a''i = ''γ''i ''C''i): : Q_r = \frac = \frac The Nernst equation is a function of and can be written as follows: E_\text = E^\ominus_\text - \frac \ln Q_r=E^\ominu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nernst Equation
In electrochemistry, the Nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction ( half-cell or full cell reaction) from the standard electrode potential, absolute temperature, the number of electrons involved in the redox reaction, and activities (often approximated by concentrations) of the chemical species undergoing reduction and oxidation respectively. It was named after Walther Nernst, a German physical chemist who formulated the equation. Expression General form with chemical activities When an oxidized species () accepts a number ''z'' of electrons () to be converted in its reduced form (), the half-reaction is expressed as: : Ox + ze- -> Red The reaction quotient ('), also often called the ion activity product (''IAP''), is the ratio between the chemical activities (''a'') of the reduced form (the reductant, ) and the oxidized form (the oxidant, ). The chemical activity of a dissolved spe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |