|
Ethylmercury Chloride
Ethylmercury (sometimes ethyl mercury) is a cation composed of an organic CH3CH2— species (an ethyl group) bound to a mercury(II) centre, making it a type of organometallic cation, and giving it a chemical formula C2H5Hg+. The main source of ethylmercury is thimerosal. Synthesis and structure Ethylmercury (C2H5Hg+) is a substituent of compounds: it occurs as a component of compounds of the formula C2H5HgX where X = chloride, thiolate, or another organic group. Most famously X = the mercaptide group of thiosalicylic acid as in thiomersal. In the body, ethylmercury is most commonly encountered as derivatives with a thiolate attached to the mercury. In these compounds, Hg(II) has a linear or sometimes trigonal coordination geometry. Given the comparable electronegativities of mercury and carbon, the mercury-carbon bond is described as covalent. Toxicity The toxicity of ethylmercury is well studied. Like methylmercury, ethylmercury distributes to all body tissues, crossing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomercury Compounds
Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Many organomercury compounds are highly toxic, but some are used in medicine, e.g., merbromin ("Mercurochrome") and the vaccine preservative thiomersal. Structure and bonding Most organomercury compounds feature diamagnetic Hg(II) and adopt a linear C-Hg-X structure. Indeed, no organic derivatives of Hg are known, as Hg requires electronegative subtituents for condensed-phase stability. Hg(II) derivatives are neither Lewis basic or Lewis acidic. They are stable to oxygen and water, indicating the low polarity of the Hg-C bond. Mercury forms a compound with two cyclopentadiene ligands, but the resulting complex may not be a metallocene. When made in the 1950s, it was too sensitive for structural determination. Toxicity The toxicity of organomercury compounds presents both dangers and benefits. Dimethylmercury in particular is notoriously toxic, but found use as an antifungal ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Poisoning
Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type, dose, method, and duration of exposure. They may include muscle weakness, poor coordination, numbness in the hands and feet, skin rashes, anxiety, memory problems, trouble speaking, trouble hearing, or trouble seeing. High-level exposure to methylmercury is known as Minamata disease. Methylmercury exposure in children may result in acrodynia (pink disease) in which the skin becomes pink and peels. Long-term complications may include kidney problems and decreased intelligence. The effects of long-term low-dose exposure to methylmercury are unclear. Forms of mercury exposure include metal, vapor, salt, and organic compound. Most exposure is from eating fish, amalgam-based dental fillings, or exposure at a workplace. In fish, those higher up in the food chain generally have higher levels of mercury, a process known as biomagnification. Less commonly, poisoning may occu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylmercury
Diethylmercury is a flammable, colorless liquid, and one of the strongest known neurotoxins. This organomercury compound is described as having a slightly sweet smell, though inhaling enough fumes to notice this would be hazardous. This chemical can cross the blood–brain barrier, causing permanent brain damage. It is, however, considerably less toxic than dimethylmercury. The resulting (CH3CH2)2Hg is a dense liquid (2.466 g/cm3) that boils at 57 °C at 16 torr. This extremely toxic compound is slightly soluble in ethanol and soluble in ether. Synthesis Diethylmercury can be obtained from the reaction between ethylmagnesium bromide and mercury(II) chloride. :2 C2H5MgBr + HgCl2 → Hg(C2H5)2 + MgBr2 + MgCl2 Other methods are also known. See also * Ethylmercury * Mercury poisoning Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type, dose, method, and duration of exposure. They may include muscle weakness, poor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half-life
Half-life is a mathematical and scientific description of exponential or gradual decay. Half-life, half life or halflife may also refer to: Film * Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang * ''Half Life: A Parable for the Nuclear Age'', a 1985 Australian documentary film Literature * Half Life (Jackson novel), ''Half Life'' (Jackson novel), a 2006 novel by Shelley Jackson * Half-Life (Krach novel), ''Half-Life'' (Krach novel), a 2004 novel by Aaron Krach * Halflife (Michalowski novel), ''Halflife'' (Michalowski novel), a 2004 novel by Mark Michalowski * ''Rozpad połowiczny'' (), a 1988 award-winning dystopia novel by Edmund Wnuk-Lipiński Music *Half Life (3 album), ''Half Life'' (3 album) (2001) *Halflife (EP), ''Halflife'' (EP), an EP by Lacuna Coil and the title track *''Half-Life E.P.'', an EP by Local H * "Half Life", a song by 10 Years from ''The Autumn Effect'' * "Half Life", a song by Come from ''Near-Life Experience'' * "Ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
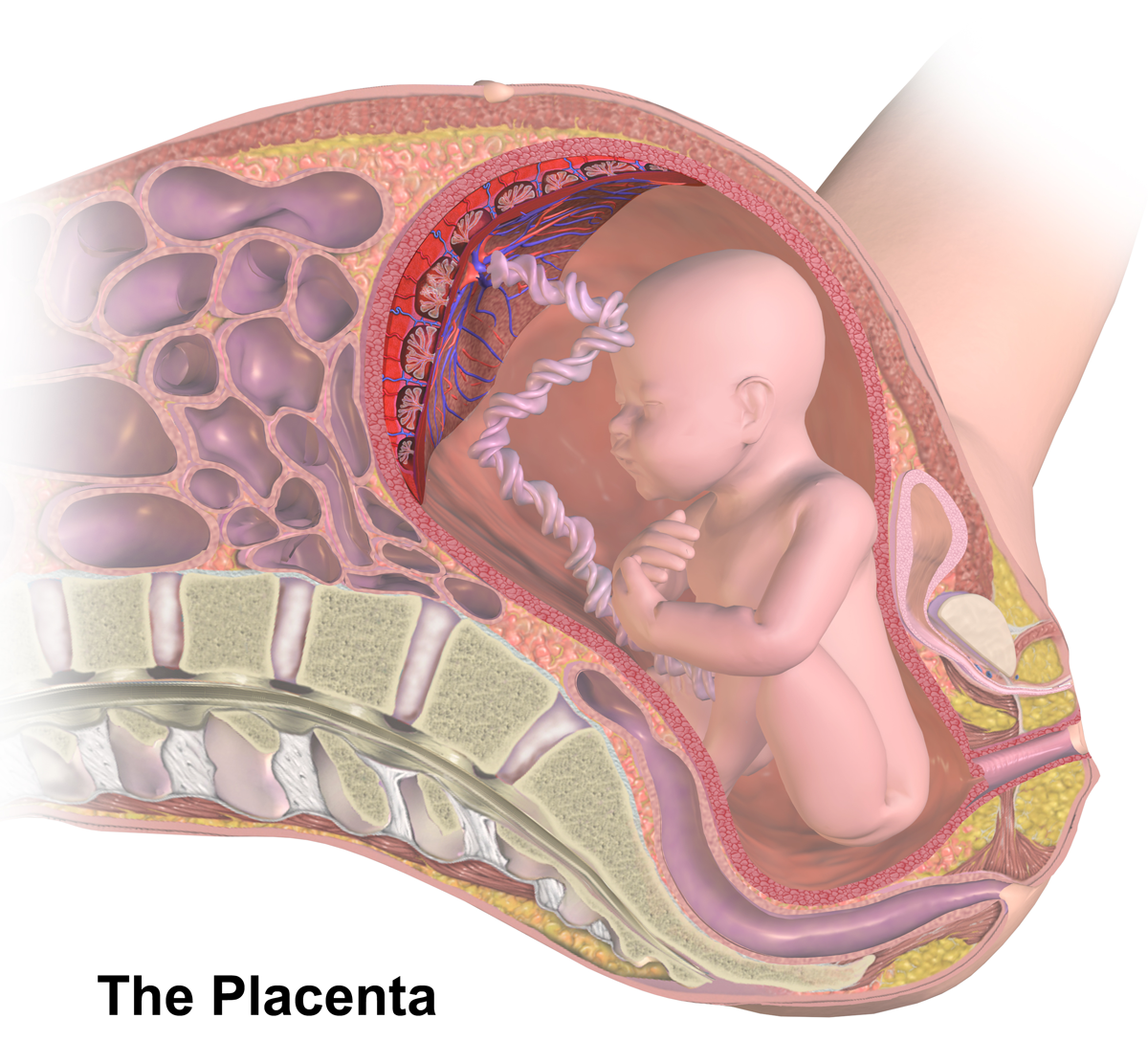
Placental Barrier
The placenta (: placentas or placentae) is a temporary embryo, embryonic and later Fetus, fetal organ (biology), organ that begins embryonic development, developing from the blastocyst shortly after implantation (embryology), implantation. It plays critical roles in facilitating nutrient, gas, and waste exchange between the physically separate maternal and fetal circulations, and is an important Endocrine system, endocrine organ, producing hormones that regulate both Maternal physiological changes in pregnancy, maternal and fetal physiology during pregnancy. The placenta connects to the fetus via the umbilical cord, and on the opposite aspect to the maternal uterus in a species-dependent manner. In humans, a thin layer of maternal decidual (Endometrium, endometrial) tissue comes away with the placenta when it is expelled from the uterus following birth (sometimes incorrectly referred to as the 'maternal part' of the placenta). Placentas are a defining characteristic of placental m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood–brain Barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that regulates the transfer of solutes and chemicals between the circulatory system and the central nervous system, thus protecting the brain from harmful or unwanted substances in the blood. The blood–brain barrier is formed by endothelial cells of the Capillary, capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive transport, passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function. The blood–brain barrier restricts the passage of pathogens, the diffusion of solutes in the blood, and Molecular mass, large or Hydrophile, hydrophilic molecules into the cerebrospinal fluid, while a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmercury
Methylmercury is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a bioaccumulative environmental toxicant with a 50-day half-life. Methylmercury (derived biologically from dimethylmercury) is the causative agent of the infamous Minamata disease. Methylmercury is designated as a "priority hazardous substance" according to the Directive on Environmental Quality Standards (Directive 2013/39/EU). Structure and chemistry "Methylmercury" is a shorthand for the hypothetical "methylmercury cation", sometimes written ''methylmercury(1+) cation'' or ''methylmercury(II) cation''. This functional group is composed of a methyl group Chemical bond, bonded to an atom of Mercury (element), mercury. Its chemical formula is (sometimes written as ). The Methylmercury compound has an overall charge of +1, with Hg in the +2 oxidation state. Methylmer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiomersal
Thiomersal (International Nonproprietary Name, INN), or thimerosal (United States Adopted Name, USAN, Japanese Accepted Name, JAN), also sold under the name merthiolate, is an organomercury compound. It is a well-established antiseptic and antifungal agent. The pharmaceutical corporation Eli Lilly and Company named it Merthiolate. It has been used as a preservative in vaccines, immunoglobulin preparations, Allergy#Diagnosis, skin test antigens, antivenins, ophthalmology, ophthalmic and nasal products, and tattoo inks. In spite of the scientific consensus that fears about its safety are unsubstantiated, its use as a vaccine preservative has been Thiomersal controversy, called into question by vaccine hesitancy, anti-vaccination groups. A 1999 statement issued in Centers for Disease Control and Prevention, CDC's Morbidity and Mortality Weekly Report announced that "the Public Health Service (United States Public Health Service Commissioned Corps, PHS), the American Academy of Ped ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiosalicylic Acid
Thiosalicylic acid is an organosulfur compound containing carboxyl and sulfhydryl functional groups. Its molecular formula is ''ortho''-. It is a yellow solid that is slightly soluble in water, ethanol and diethyl ether, and alkanes, but more soluble in DMSO. Preparation Thiosalicylic acid can be prepared from anthranilic acid via diazotization followed by the addition of sodium sulfide and then reduction with zinc. Uses Thiosalicylic acid is a precursor to the dyestuff thioindigo. It is also used to make the vaccine preservative thiomersal. It is a precursor to drug candidates for treatment of atherosclerosis and melanoma. The preservative benzisothiazolinone Benzisothiazolinone (BIT) is an organic compound with the formula C6H4SN(H)CO. A white solid, it is structurally related to isothiazole, and is part of a class of molecules called isothiazolinones. BIT is widely used as a preservative and antimicr ... is prepared from thiosalicylic acid. References {{Reflist Benzoic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |