|
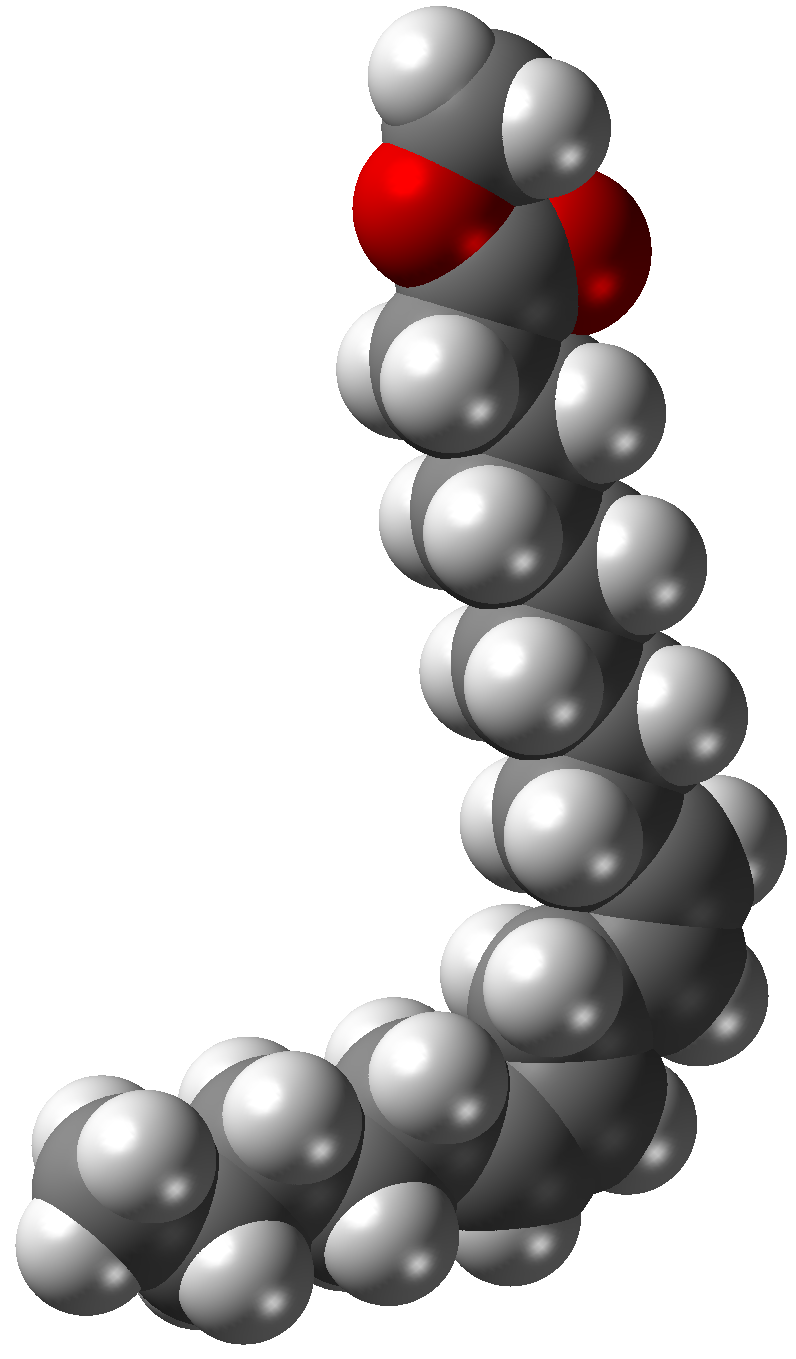
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Historically, vinegar was produced from the third century BC and was likely the first acid to be produced in large quantities. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is funda ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g., alkyl, alkenyl, aryl), or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They often have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid () is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trithiocarbonate
Thiocarbonate describes a family of anions with the general chemical formula (''x'' = 0, 1, or 2): *for ''x'' = 2 it is monothiocarbonate ion *for ''x'' = 1 it is dithiocarbonate ion *for ''x'' = 0 it is trithiocarbonate ion Like the carbonate dianion, the thiocarbonate ions are Trigonal planar molecular geometry, trigonal planar, with carbon atom at the center of triangle, and oxygen and sulfur atoms at the peaks of the triangle. The average bond order between C and S or O is . The state of protonation is usually not specified. These anions are good nucleophiles and good ligands. Thiocarbonates refer to Salt (chemistry), salts of those ions as well (e.g. potassium trithiocarbonate, ). Thiocarbonates refer to esters of those ions as well (e.g. dimethyl trithiocarbonate, ). They contain trigonal planar divalent functional groups similar to these anions (''x'' = 0, 1, or 2, R is organyl group). Esters with the formula are also called xanthates, while esters with the formula are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactone
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated. Lactones are formed by lactonization, the intramolecular esterification of the corresponding hydroxycarboxylic acids. Nomenclature Greek alphabet#Letters, Greek prefixes in alphabetical order indicate ring size. Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α-lactone = 3-membered ring, β-lac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology. Lipids are broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/ unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condensatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acid Ester
Fatty acid esters (FAEs) are a type of ester that result from the combination of a fatty acid with an alcohol. When the alcohol component is glycerol, the fatty acid esters produced can be monoglycerides, diglycerides, or triglycerides. Dietary fats are chemically triglycerides. Esters of fatty acids are colorless, although degraded samples are sometime appear to be yellow or even brown. The triglycerides are powders, flakes, coarse powders, or granular or waxy lumps, oils or liquids. They are almost odorless. Biodiesels are typically fatty acid esters made by the transesterification of vegetable fats and oils. In this process the glycerol component is replaced with a different alcohol. The most commonly used alcohol is methanol, producing fatty acid methyl esters (FAME). When ethanol is used fatty acid ethyl esters (FAEE) are created. Other alcohols used for the production of biodiesel include butanol and isopropanol. Fatty acid ethyl esters are biomarkers for the consumption ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amides
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amino group (); or, equivalently, an acyl (alkanoyl) group () joined to an amino group. Common amides are formamide (), acetamide (), benzamide (), and dimethylformamide (). Some uncommon examples of amides are ''N''-chloroacetamide () and chloroformamide (). Amides are qualified as primary, secondary, and tertiary according to the number of acyl groups bounded to the nitrogen atom. Nomenclature The core of amides is called the amide group (specifically, carboxamide group). In the usual no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters. In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis. Terminology in biochemical literature In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula . Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid. In physiology, carbon dioxide excreted by the lungs may be called ''volatile acid'' or ''respiratory acid''. Anh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxoacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid. Description Under Lavoisier's original theory, all acids contained oxygen, which was named from . It was later discovered that some acids, notably hydrochloric acid, did not contain oxygen and so acids were divided into oxo-acids and these new hydroacids. All oxyacids have the acidic hydrogen bound to an oxygen atom, so bond strength (length) is not a factor, as it is with binary nonmetal hydrides. Rather, the electronegativity of the central atom and the number of oxygen atoms determine oxyacid acidity. For oxyacids with the same central atom, acid strength increases with the number of oxygen atoms attached to it. With the same number of oxygen atoms attached to it, acid strength increases with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoric Acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non- volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Removal of all three ions gives the phosphate ion . Removal of one or two protons gives dihydrogen phosphate ion , and the hydrogen phosphate ion , respectively. Phosphoric acid forms esters, called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other " phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Production Phosphoric acid is produced industrially by one of two routes, wet processes and dry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |