|
Enzyme-hydrolyzed Vegetable Protein
Hydrolyzed vegetable protein (HVP) products are foodstuffs obtained by the hydrolysis of protein, and have a meaty, savory taste similar to broth (bouillon). Regarding the production process, a distinction can be made between acid-hydrolyzed vegetable protein (aHVP), enzymatically produced HVP, and other seasonings, e.g., fermented soy sauce. Hydrolyzed vegetable protein products are particularly used to round off the taste of soups, sauces, meat products, snacks, and other dishes, as well as for the production of ready-to-cook soups and bouillons. History Food technologists have long known that protein hydrolysis produces a meat bouillon-like odor and taste. Hydrolysates have been a part of the human diet for centuries, notably in the form of fermented soy sauce, or Shoyu. Shoyu, traditionally made from wheat and soy protein, has been produced in Japan for over 1,500 years, following its introduction from mainland China. The origins of producing these materials through the aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteases
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in numerous biological pathways, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Classification Based on catalytic residue Proteases can be classified into seven broad groups: * Serine proteases - using a serine alcohol * Cysteine prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-MCPD
3-MCPD (3-monochloropropane-1,2-diol or 3-chloropropane-1,2-diol) is an organic chemical compound with the formula HOCH2CH(OH)CH2Cl. It is a colorless liquid. The compound has attracted notoreity as the most common member of chemical food contaminants known as chloropropanols. It is suspected to be carcinogenic in humans. Accidental and intentional production 3-MCPD, together with its isomer 2-MCPD, is thought to be produced when fat-containing foods are treated at high temperatures with hydrochloric acid. Such treatments are sometimes used to accelerate protein hydrolysis, making food more digestable. In such a treatment chloride is thought to react with the glycerol backbone of lipids to produce 3-MCPD and 2-MCPD. Chlorination of glycerol gives the 3-MCPD: : The same compound can be produced by hydrolysis of epichlorohydrin. Occurrence In 2009, 3-MCPD was found in some East Asian and Southeast Asian sauces such as oyster sauce, Hoisin sauce, and soy sauce. Using h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lovage
Lovage ( ; ''Levisticum officinale'') is a perennial plant, the sole species in the genus ''Levisticum'' in the family Apiaceae, subfamily Apioideae. It has been long cultivated in Europe and the leaves are used as a herb, the roots as a vegetable, and the seeds as a spice, especially in southern European cuisine. Its flavour and smell are reminiscent both of celery and parsley, only more intense and spicier than either. The seeds can be used in the same way as fennel seeds. Description Lovage is an erect, herbaceous, perennial plant growing to tall, with a basal rosette of leaves and stems with further leaves, the flowers being produced in umbels at the top of the stems. The stems and leaves are shiny glabrous green to yellow-green and smell somewhat similar to celery when crushed. The larger basal leaves are up to long, tripinnate, with broad triangular to rhomboidal, acutely pointed leaflets with a few marginal teeth; the stem leaves are smaller, and less divided with few ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutaminase
Glutaminase (, ''glutaminase I'', ''L-glutaminase'', ''glutamine aminohydrolase'') is an amidohydrolase enzyme that generates glutamate from glutamine. Glutaminase has tissue-specific isoenzymes. Glutaminase has an important role in glial cells. Glutaminase catalyzes the following reaction: :Glutamine + → glutamate + It is useful in the food industry, as it converts free glutamine into the umami-tasting free glutamate. Tissue distribution Glutaminase is expressed and active in periportal hepatocytes, where it generates ammonium for urea synthesis, as does glutamate dehydrogenase. Glutaminase is also expressed in the epithelial cells of the renal tubules, where the produced ammonia is excreted as ammonium ions. This excretion of ammonium ions is an important mechanism of renal acid-base regulation. During chronic acidosis, glutaminase is induced in the kidney, which leads to an increase in the amount of ammonium ions excreted. Glutaminase can also be found in the intesti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Umami
Umami ( from ), or savoriness, is one of the five basic tastes. It is characteristic of broths and cooked meats. People taste umami through taste receptors that typically respond to glutamates and nucleotides, which are widely present in meat broths and fermented products. Glutamates are commonly added to some foods in the form of monosodium glutamate (MSG), and nucleotides are commonly added in the form of disodium guanylate, inosine monophosphate (IMP) or guanosine monophosphate (GMP). Since umami has its own receptors rather than arising out of a combination of the traditionally recognized taste receptors, scientists now consider umami to be a distinct taste. Foods that have a strong umami flavor include meats, shellfish, fish (including fish sauce and preserved fish such as Maldives fish, '' katsuobushi'', sardines, and anchovies), '' dashi'', tomatoes, mushrooms, hydrolyzed vegetable protein, meat extract, yeast extract, kimchi, cheeses, and soy sauce. In 1908, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxypeptidase D
Carboxypeptidase D can refer to one of several enzymes. A family of serine carboxypeptidases (i.e. enzymes that use an active site serine residue) includes (, ''cereal serine carboxypeptidase II'', ''Saccharomyces cerevisiae KEX1 gene product'', ''carboxypeptidase Kex1'', ''gene KEX1 serine carboxypeptidase'', ''KEX1 carboxypeptidase'', ''KEX1 proteinase'', ''KEX1DELTAp'', ''CPDW-II'', ''serine carboxypeptidase'', ''Phaseolus proteinase'') is an enzyme. This enzyme has an optimal pH of 4.5-6.0, is inhibited by diisopropyl fluorophosphate, and catalyses the following chemical reaction : Preferential release of a C-terminal arginine or lysine residue A completely distinct enzyme has also been named carboxypeptidase D (EC number 3.4.17.22). This second enzyme is a metallocarboxypeptidase (i.e. uses a zinc ion in the active site instead of a serine residue) and is broadly expressed in mammalian tissues. Like the serine carboxypeptidase, the metallocarboxypeptidase D also removes C-te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
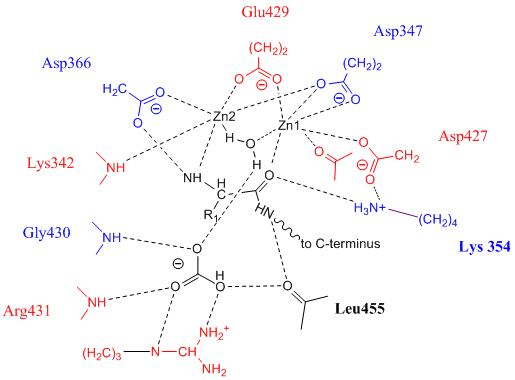
Leucine Aminopeptidase
Leucyl aminopeptidases (, ''leucine aminopeptidase'', ''LAPs'', ''leucyl peptidase'', ''peptidase S'', ''cytosol aminopeptidase'', ''cathepsin III'', ''L-leucine aminopeptidase'', ''leucinaminopeptidase'', ''leucinamide aminopeptidase'', ''FTBL proteins'', ''proteinates FTBL'', ''aminopeptidase II'', ''aminopeptidase III'', ''aminopeptidase I'') are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, ''Escherichia coli'' (''E. coli'') LAP (also known as PepA or XerB), and the solanaceous-specific acidic LAP (LAP-A) in tomato (''Solanum lycopersicum''). Enzyme description, structure, and active site The active sites in PepA and in bovine lens LAP have been found to be similar. Shown in the picture below is the proposed model for the active site of LAP-A in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exopeptidase
An exopeptidase is any peptidase that catalyzes the cleavage of the terminal (or the penultimate) peptide bond; the process releases a single amino acid, dipeptide or a tripeptide from the peptide chain. Depending on whether the amino acid is released from the amino or the carboxy terminal (N-terminus or C-terminus), an exopeptidase is further classified as an aminopeptidase or a carboxypeptidase, respectively. Thus, an aminopeptidase, an enzyme in the brush border of the small intestine, will cleave a single amino acid from the amino terminal, whereas carboxypeptidase, which is a digestive enzyme present in pancreatic juice, will cleave a single amino acid from the carboxylic end of the peptide. Some examples of exopeptidases include: * Carboxypeptidase A - cleaves C-terminal Phe, Tyr, Trp, or Leu * Carboxypeptidase B - cleaves C-terminal Lys or Arg * Aminopeptidase - cleaves any N-terminal amino acid * Prolinase - cleaves N-terminal Pro from dipeptides * Prolidase - cleav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcalase
Subtilisin is a protease (a protein-digesting enzyme) initially obtained from ''Bacillus subtilis''. Subtilisins belong to subtilases, a group of serine proteases that – like all serine proteases – initiate the nucleophilic attack on the peptide (amide) bond through a serine residue at the active site. Subtilisins typically have molecular weights 27kDa. They can be obtained from certain types of soil bacteria, for example, '' Bacillus amyloliquefaciens'' from which they are secreted in large amounts. Nomenclature "Subtilisin" does not refer to a single protein, but to an entire clade under subtilases containing the classical subtilisins. The clade can be further divided into four groups: "true subtilisins" (containing the classical members), "high-alkaline subtilisins", "intracellular subtilisins", and "phylogenetically intermediate subtilisins" (PIS). Notable subtilisins include: Other non-commercial names include ''ALK-enzyme'', ''bacillopeptidase'', ''Bacillus sub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endopeptidase
Endopeptidase or endoproteinase are proteolytic peptidases that break peptide bonds of nonterminal amino acids (i.e. within the molecule), in contrast to exopeptidases, which break peptide bonds from end-pieces of terminal amino acids. For this reason, endopeptidases cannot break down peptides into monomers, while exopeptidases can break down proteins into monomers. A particular case of endopeptidase is the oligopeptidase, whose substrates are oligopeptides instead of proteins. They are usually very specific for certain amino acids. Examples of endopeptidases include: * Trypsin - cuts after Arg or Lys, unless followed by Pro. Very strict. Works best at pH 8. * Chymotrypsin - cuts after Phe, Trp, or Tyr, unless followed by Pro. Cuts more slowly after His, Met or Leu. Works best at pH 8. * Elastase - cuts after Ala, Gly, Ser, or Val, unless followed by Pro. * Thermolysin - cuts ''before'' Ile, Met, Phe, Trp, Tyr, or Val, unless ''preceded'' by Pro. Sometimes cuts after Ala, Asp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |