|
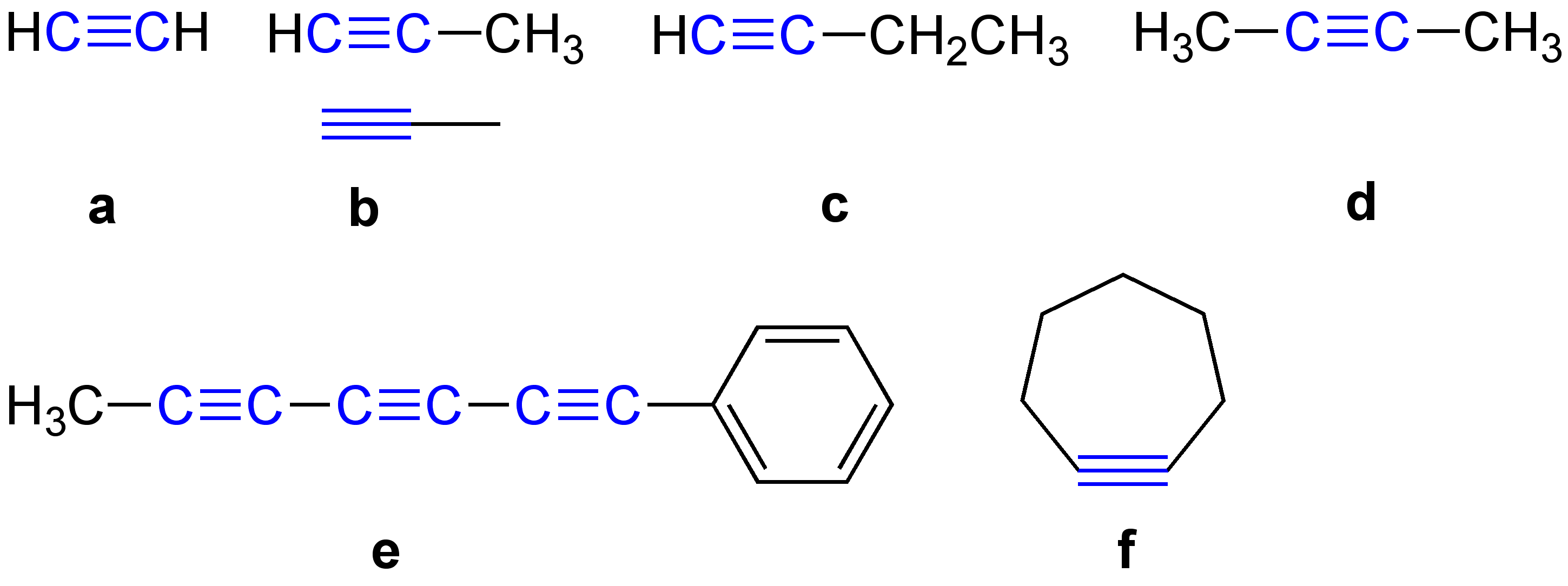
Enyne
An enyne is an organic compound containing a double bond (alkene) and a triple bond (alkyne). It is called a conjugated enyne when the double and triple bonds are conjugated. The term is a contraction of the terms alkene and alkyne. The simplest enyne is vinylacetylene. Some examples of enynes found in nature are isanolic acid and exocarpic acid. See also *Enyne metathesis * Enediyne *Polyyne A polyyne is any organic compound with alternating Single bond, single and triple bonds; that is, a series of consecutive alkynes, with ''n'' greater than 1. These compounds are also called polyacetylenes, especially in the natural products and ... References {{organic-chemistry-stub Chemical nomenclature Alkenes Alkynes Conjugated hydrocarbons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enyne Metathesis
An enyne metathesis is an organic reaction taking place between an alkyne and an alkene with a metal carbene catalyst forming a butadiene. This reaction is a variation of olefin metathesis. The general scheme is given by ''scheme 1'': : When the reaction is Intramolecular reaction, intramolecular (in an enyne) it is called ring-closing enyne metathesis or RCEYM (''scheme 2''): : with Y representing oxygen or nitrogen and n an integer. The reaction was first described in 1985 with the conversion of biphenyl 3.1 to a phenanthrene in ''scheme 3'': : The carbene is a tungsten metal carbonyl, carbonyl when used in stoichiometric amounts (1 equivalent) yields 41% of the phenanthrene 3.2 and when used in catalytic amounts phenanthrene 3.3. The stereoselectivity of this reaction is large with the metal atom exclusively adding to one of the alkyne carbon atoms in the initial reaction step. Reaction mechanism The reaction mechanism for this reaction is outlined in scheme 4: : In the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinylacetylene
Vinylacetylene is the organic compound with the formula or . The colourless gas was once used in the polymer industry. It is composed of both alkyne and alkene groups and is the simplest enyne. Safety Vinylacetylene is extremely dangerous because in high enough concentrations (typically > 30 mole percent, but pressure dependent) it can auto-detonate (explode without air being present) especially at elevated pressures, such as those seen in chemical plants processing C4 hydrocarbons (hydrocarbons with 4 carbon atoms). An example of such an explosion occurred at a Union Carbide plant in Texas City in 1969. Synthesis Vinylacetylene was first synthesized by Hofmann elimination of the related quaternary ammonium salt: : It is usually synthesized by dehydrohalogenation of 1,3-dichloro-2-butene . It also arises via the dimerization of acetylene, which is catalyzed by copper(I) chloride. Dehydrogenation of 1,3-butadiene is yet another route. Application At one time, chloroprene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isanolic Acid
Isanolic acid is a linear fatty acid composed of 18 carbon atoms, with two triple bonds in the positions 9≡10 and 11≡12, a double bond in the position 17=18, and a hydroxyl-OH in the position 8. The acid is one of the rare polyacetylenic acids with conjugated triple bonds. The compound belongs to the family of diynes and enynes, as well as to the alkyne and alkenoic acids. The related compounds are isanic and ketoisanic acids, both containing only one hydroxyl group. Discovery The acid was initially isolated in 1937 by researchers A. Steger and J. van Loon from the seed oilof the tree '' Ongokea gore'' or ''Ongokea klaineana'', a plant from equatorial Africa, called in the native language "boleka" or "isano"; a common name of isanolic acid comes from the latter. They also discovered isanic acid. The oil seeds contain about 60% lipids. Since the compound is hard to isolate, various analyses have revealed non-homogeneous data on the concentration of isanolic acid in isano o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enediyne
Enediynes are organic compounds containing two triple bonds and one double bond. Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne anticancer antibiotics). They are efficient at inducing apoptosis in cells, but cannot differentiate cancerous cells from healthy cells. Consequently, research is being conducted to increase the specificity of enediyne toxicity. Structure and reactivity A nine- or ten-membered ring containing a double bond between two triple bonds is termed the warhead of the enediyne. In this state, the warhead is inactive. Enediynes are triggered into a chemically active state via Bergman or Myers-Saito cyclization. The triggering mechanism can be attributed to an intramolecular nucleophilic attack initiated by one of the variable regions of the molecule. Triggering can also occur via attack by an external nucleophile. Bergman cyclization restructures the enediyne ring into two smaller rings. One electron from each of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exocarpic Acid
Exocarpic acid is an unsaturated, conjugated fatty acid with one double bond and two triple bonds. It is isomeric to isanic acid and belongs to the class of alkyne and alkenoic acids, as well as the diynes and enynes. The acid's delta notation is 18:3-delta-9a,11a,13t. Its structural formula is CH 3(CH2)3-CH=CH-C≡CC≡C-(CH2)7-COOH. The acid was initially isolated in 1959, by H. H. Hatt and co-workers, from the roots of the Australian tree ''Exocarpos cupressiformis''; from this genus they derived the common name of exocarpic acid. Natural occurrence Exocarpic acid is found in various plant species of the order ''Santalales''. In the family ''Olacaceae'', it occurs, among others, in the seed oils of ''Curupira tefeensis'' and ''Olax dissitiflora''. In the family ''Santalaceae'', it occurs in several species of the genus ''Thesium'', such as mountain flax and ''Thesium chinense'', as well as in several species of the genus ''Buckleya'', such as ''Buckleya lanceolata'' and ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkynes
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Calgary
{{Infobox university , name = University of Calgary , image = University of Calgary coat of arms without motto scroll.svg , image_size = 150px , caption = Coat of arms , former_name = Normal School (1905–1913)Calgary Normal School (1913–1945)Calgary Branch of the Faculty of Education of the University of Alberta (1945–1958)University of Alberta in Calgary (1958–1966){{efn, The following are names of the predecessor institution which the University of Calgary originates from, prior to its reorganization as a standalone university. , motto = {{Lang, gd, Mo Shùile Togam Suas (Canadian Gaelic, Gaelic) , mottoeng = I will lift up my eyes , established = {{Start date and age, 1966, 04, 26, df=yes, p=yes, br=yes , type = Public university, Public , endowment = {{CAD, 1.176 billion (2023) , chancellor = J ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated System
In physical organic chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases Chemical stability, stability. It is Resonance (chemistry), conventionally represented as having alternating single and multiple covalent bond, bonds. Lone pairs, radical (chemistry), radicals or carbenium ions may be part of the system, which may be Cyclic molecule, cyclic, acyclic, Linear molecular geometry, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele (chemist), Johannes Thiele. Conjugation is the orbital overlap, overlap of one p-orbital with another across an adjacent Sigma bond, σ bond (in transition metals, d-orbitals can be involved). A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of pi el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, single bonds or double bond, double bonds, with a bond order of three. The most common triple bond is in a nitrogen N2 molecule; the second most common is that between two carbon atoms, which can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as diphosphorus and carbon monoxide, are also triple bonded. In skeletal formula, skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms. Bonding Triple bonding can be explained in terms of orbital hybridization. In the case of acetylene, each carbon atom has two sp orbital, sp-orbitals and two p-orbitals. The two sp-orbitals are linear, with 180° bond angles, and occupy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |