|
Enterocin
Enterocin and its derivatives are bacteriocins synthesized by the lactic acid bacteria, ''Enterococcus''. This class of polyketide antibiotics are effective against foodborne pathogens including '' L. monocytogenes, Listeria,'' and ''Bacillus.'' Due to its proteolytic degradability in the gastrointestinal tract, enterocin is used for controlling foodborne pathogens via human consumption. History Enterocin was discovered from soil and marine ''Streptomyces'' strains as well as from marine ascidians of ''Didemnum'' and it has also been found in a mangrove strains ''Streptomyces qinglanensis'' and '' Salinispora pacifica''. Total synthesis The total synthesis of enterocin has been reported. Biosynthesis Enterocin has a caged, tricyclic, nonaromatic core and its formation undergoes a flavoenzyme (EncM) catalyzed Favorskii-like rearrangement of a poly(beta-carbonyl). Studies done on enterocin have shown that it is biosynthesized from a type II polyketide synthase (PKS) pathway, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteriocin
Bacteriocins are proteinaceous or peptide, peptidic toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strain(s). They are similar to yeast and paramecium killing factors, and are structurally, functionally, and ecologically diverse. Applications of bacteriocins are being tested to assess their application as narrow-spectrum antibiotics. Bacteriocins were first discovered by André Gratia in 1925. He was involved in the process of searching for ways to kill bacteria, which also resulted in the development of antibiotics and the discovery of bacteriophage, all within a span of a few years. He called his first discovery a ''colicine'' because it was made by ''Escherichia coli, E. coli.'' Classification Bacteriocins are categorized in several ways, including producing strain, common resistance mechanisms, and mechanism of killing. There are several large categories of bacteriocin which are only phenomenologically related. These include t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoic Acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which is used for benzyl), thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source. Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates (). History Benzoic acid was discovered in the sixteenth century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compounds With 3 Rings
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactones
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated. Lactones are formed by lactonization, the intramolecular esterification of the corresponding hydroxycarboxylic acids. Nomenclature Greek prefixes in alphabetical order indicate ring size. Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α-lactone = 3-membered ring, β-lactone = 4-membered, γ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen Heterocycles
Oxygen is a chemical element; it has symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will bind covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula . Dioxygen gas currently constitutes approximately 20.95% molar fraction of the Earth's atmosphere, though this has changed considerably over long periods of time in Earth's history. A much rarer triat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. In mammals, these enzymes oxidize steroids, fatty acids, xenobiotics, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted. P450s are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived from the spectrophotometry, spectrophotometric peak at the wavelength of the absorption spectroscopy, absorption maximum of the enzyme (450 nanometre, nm) when it is in the redox, reduced state and complexed with carbon monoxide. Most P450s require a protein partner to deliver one or more electrons to reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-methyltransferase
An O-methyltransferase (OMT) is a type of methyltransferase enzyme transferring a methyl group on a molecule. Examples are : * Acetylserotonin O-methyltransferase * Apigenin 4'-O-methyltransferase * Caffeate O-methyltransferase * Caffeoyl-CoA O-methyltransferase * Catechol O-methyltransferase * Chlorophenol O-methyltransferase * Columbamine O-methyltransferase * Demethylmacrocin O-methyltransferase * 3'-demethylstaurosporine O-methyltransferase * Demethylsterigmatocystin 6-O-methyltransferase * 3-demethylubiquinone-9 3-O-methyltransferase * 3,7-dimethylquercetin 4'-O-methyltransferase * Fatty-acid O-methyltransferase * Glucuronoxylan 4-O-methyltransferase * 10-hydroxydihydrosanguinarine 10-O-methyltransferase * 12-hydroxydihydrochelirubine 12-O-methyltransferase * 6-hydroxymellein O-methyltransferase * 3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase * 8-hydroxyquercetin 8-O-methyltransferase * Iodophenol O-methyltransferase * Isobutyraldoxime O-methyltransfe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base. The reaction produces a β-keto ester or a β- diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. The reaction has often been displaced by diketene-based chemistry, which affords acetoacetic esters. Requirements At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid. Biosynthesis Malonyl-CoA cannot cross membranes and there is no known malonyl-CoA import mechanism. The biosynthesis therefore takes place locally: * cytosol: Malonyl-CoA is formed by carboxylating acetyl-CoA using the highly regulated enzyme acetyl-CoA carboxylase 1 (ACC1). One molecule of acetyl-CoA joins with a molecule of bicarbonate, requiring energy rendered from ATP. * Mitochondrial outer membrane: Malonyl-CoA is formed by carboxylating acetyl-CoA using the highly regulated enzyme acetyl-CoA carboxylase 2 (ACC2). The reaction is the same as with ACC1. * mitochondrial matrix: Malonyl-CoA is formed in coordinated fashion by mtACC1, a mitochondrial isoform of ACC1, and acyl-CoA synthetase family member 3 (ACSF3), a mitochondrial malonyl-CoA synthetase. MtACC1, like cytosolic ACC1 catalyses the carboxylation of acetyl-CoA, while ACSF3 catalyses the thioesterification of malonate to coenzyme A. The latter serves ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
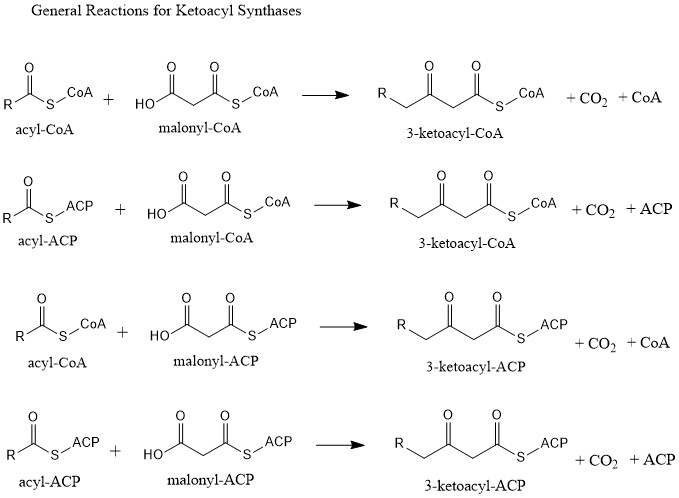
Ketoacyl Synthase
Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the resulting acyl chain is two carbon atoms longer than before. KSs exist as individual enzymes, as they do in type II fatty acid synthesis and type II polyketide synthesis, or as domains in large multidomain enzymes, such as type I fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs are divided into five families: KS1, KS2, KS3, KS4, and KS5. Multidomain enzyme systems Fatty acid synthase Fatty acid synthase (FAS) is the enzyme system involved in de novo fatty acid synthesis. FAS is an iterative multienzyme consisting of several component enzymes, one of which is ketoacyl synthase. There are two types of FASs: type I and type II. Type I FASs are highly integrated multidomain enzymes. They contain discrete functional domains resp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |