|
Energy Gap
In solid-state physics, an energy gap or band gap is an energy range in a solid where no electron states exist, i.e. an energy range where the density of states vanishes. Especially in condensed matter physics, an energy gap is often known more abstractly as a spectral gap, a term which need not be specific to electrons or solids. Band gap If an energy gap exists in the band structure of a material, it is called band gap. The physical properties of semiconductors are to a large extent determined by their band gaps, but also for insulators and metals the band structure—and thus any possible band gaps—govern their electronic properties. Superconductors For superconductors the energy gap is a region of suppressed density of states around the Fermi energy, with the size of the energy gap much smaller than the energy scale of the band structure. The superconducting energy gap is a key aspect in the theoretical description of superconductivity and thus features prominently in BC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid-state Physics
Solid-state physics is the study of rigid matter, or solids, through methods such as solid-state chemistry, quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic-scale properties. Thus, solid-state physics forms a theoretical basis of materials science. Along with solid-state chemistry, it also has direct applications in the technology of transistors and semiconductors. Background Solid materials are formed from densely packed atoms, which interact intensely. These interactions produce the mechanical (e.g. hardness and Elasticity (physics), elasticity), Heat conduction, thermal, Electrical conduction, electrical, Magnetism, magnetic and Crystal optics, optical properties of solids. Depending on the material involved and the conditions in which it was formed, the atoms may be arranged in a regular, geometric patt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
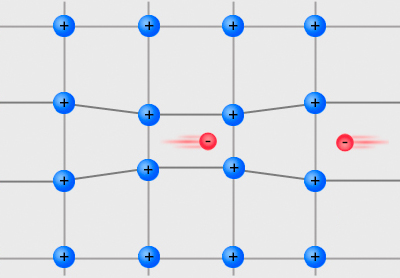
Coulomb Gap
First introduced by M. Pollak, the Coulomb gap is a soft gap in the single-particle density of states (DOS) of a system of interacting localized electrons. Due to the long-range Coulomb interactions, the single-particle DOS vanishes at the chemical potential, at low enough temperatures, such that thermal excitations do not wash out the gap. Theory At zero temperature, a classical treatment of a system gives an upper bound for the DOS near the Fermi energy, first suggested by Efros and Shklovskii. The argument is as follows: Let us look at the ground state configuration of the system. Defining E_i as the energy of an electron at site i , due to the disorder and the Coulomb interaction with all other electrons (we define this both for occupied and unoccupied sites), it is easy to see that the energy needed to move an electron from an occupied site i to an unoccupied site j is given by the expression: :\Delta E=E_j-E_i-e^2/r_ . The subtraction of the last term accounts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-temperature Superconductivity
High-temperature superconductivity (high-c or HTS) is superconductivity in materials with a critical temperature (the temperature below which the material behaves as a superconductor) above , the boiling point of liquid nitrogen. They are "high-temperature" only relative to previously known superconductors, which function only closer to absolute zero. The first high-temperature superconductor was discovered in 1986 by IBM researchers Georg Bednorz and K. Alex Müller. Although the critical temperature is around , this material was modified by Ching-Wu Chu to make the first high-temperature superconductor with critical temperature . Bednorz and Müller were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-c materials are type-II superconductors. The major advantage of high-temperature superconductors is that they can be cooled using liquid nitrogen, in contrast to previously known s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudogap
In condensed matter physics, a pseudogap describes a state where the Fermi surface of a material possesses a partial energy gap, for example, a band structure state where the Fermi surface is gapped only at certain points. The term pseudogap was coined by Nevill Mott in 1968 to indicate a minimum in the density of states at the Fermi level, ''N''(''E''F), resulting from Coulomb repulsion between electrons in the same atom, a band gap in a disordered material or a combination of these. In the modern context pseudogap is a term from the field of high-temperature superconductivity which refers to an energy range (normally near the Fermi level) which has very few states associated with it. This is very similar to a true 'gap', which is an energy range that contains no allowed states. Such gaps open up, for example, when electrons interact with the lattice. The pseudogap phenomenon is observed in a region of the phase diagram generic to cuprate high-temperature superconductors, exi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the molar gas constant, in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating Johnson–Nyquist noise, thermal noise in resistors. The Boltzmann constant has Dimensional analysis, dimensions of energy divided by temperature, the same as entropy and heat capacity. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 revision of the SI, the Boltzmann constant is one of the seven "Physical constant, defining constants" that have been defined so as to have exact finite decimal values in SI units. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly joules per kelvin, with the effect of defining the SI unit ke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cooper Pair
In condensed matter physics, a Cooper pair or BCS pair (Bardeen–Cooper–Schrieffer pair) is a pair of electrons (or other fermions) bound together at low temperatures in a certain manner first described in 1956 by American physicist Leon Cooper. Description Cooper showed that an arbitrarily small attraction between electrons in a metal can cause a paired state of electrons to have a lower energy than the Fermi energy, which implies that the pair is bound. In conventional superconductors, this attraction is due to the electron–phonon interaction. The Cooper pair state is responsible for superconductivity, as described in the BCS theory developed by John Bardeen, Leon Cooper, and John Schrieffer for which they shared the 1972 Nobel Prize in Physics. Although Cooper pairing is a quantum effect, the reason for the pairing can be seen from a simplified classical explanation. An electron in a metal normally behaves as a free particle. The electron is repelled from other electrons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BCS Theory
In physics, the Bardeen–Cooper–Schrieffer (BCS) theory (named after John Bardeen, Leon Cooper, and John Robert Schrieffer) is the first microscopic theory of superconductivity since Heike Kamerlingh Onnes's 1911 discovery. The theory describes superconductivity as a microscopic effect caused by a condensation of Cooper pairs. The theory is also used in nuclear physics to describe the pairing interaction between nucleons in an atomic nucleus. It was proposed by Bardeen, Cooper, and Schrieffer in 1957; they received the Nobel Prize in Physics for this theory in 1972. History Rapid progress in the understanding of superconductivity gained momentum in the mid-1950s. It began with the 1948 paper, "On the Problem of the Molecular Theory of Superconductivity", where Fritz London proposed that the phenomenological London equations may be consequences of the coherence of a quantum state. In 1953, Brian Pippard, motivated by penetration experiments, proposed that this would mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Energy
The Fermi energy is a concept in quantum mechanics usually referring to the energy difference between the highest and lowest occupied single-particle states in a quantum system of non-interacting fermions at absolute zero temperature. In a Fermi gas, the lowest occupied state is taken to have zero kinetic energy, whereas in a metal, the lowest occupied state is typically taken to mean the bottom of the conduction band. The term "Fermi energy" is often used to refer to a different yet closely related concept, the Fermi ''level'' (also called electrochemical potential).The use of the term "Fermi energy" as synonymous with Fermi level (a.k.a. electrochemical potential) is widespread in semiconductor physics. For example:''Electronics (fundamentals And Applications)''by D. Chattopadhyay''Semiconductor Physics and Applications''by Balkanski and Wallis. There are a few key differences between the Fermi level and Fermi energy, at least as they are used in this article: * The Fermi ener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Introduction To Solid State Physics (Kittel Book)
''Introduction to Solid State Physics'', known colloquially as ''Kittel'', is a classic condensed matter physics textbook written by American physicist Charles Kittel in 1953. The book has been highly influential and has seen widespread adoption; Marvin L. Cohen remarked in 2019 that Kittel's content choices in the original edition played a large role in defining the field of solid-state physics. It was also the first proper textbook covering this new field of physics. The book is published by John Wiley and Sons and, as of 2018, it is in its ninth edition and has been reprinted many times as well as translated into over a dozen languages, including Chinese, French, German, Hungarian, Indonesian, Italian, Japanese, Korean, Malay, Romanian, Russian, Spanish, and Turkish. In some later editions, the eighteenth chapter, titled ''Nanostructures'', was written by Paul McEuen. Along with its competitor ''Ashcroft and Mermin'', the book is considered a standard textbook in condensed m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to the energy difference (often expressed in electronvolts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote an electron from the valence band to the conduction band. The resulting conduction-band electron (and the electron hole in the valence band) are free to move within the crystal lattice and serve as charge carriers to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping levels are present in the same crystal, they form a semiconductor junction. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called " metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical element for fabricating most electronic circuits. Semiconductor devices can display a range of different useful properties, such as passing current more easil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |