|
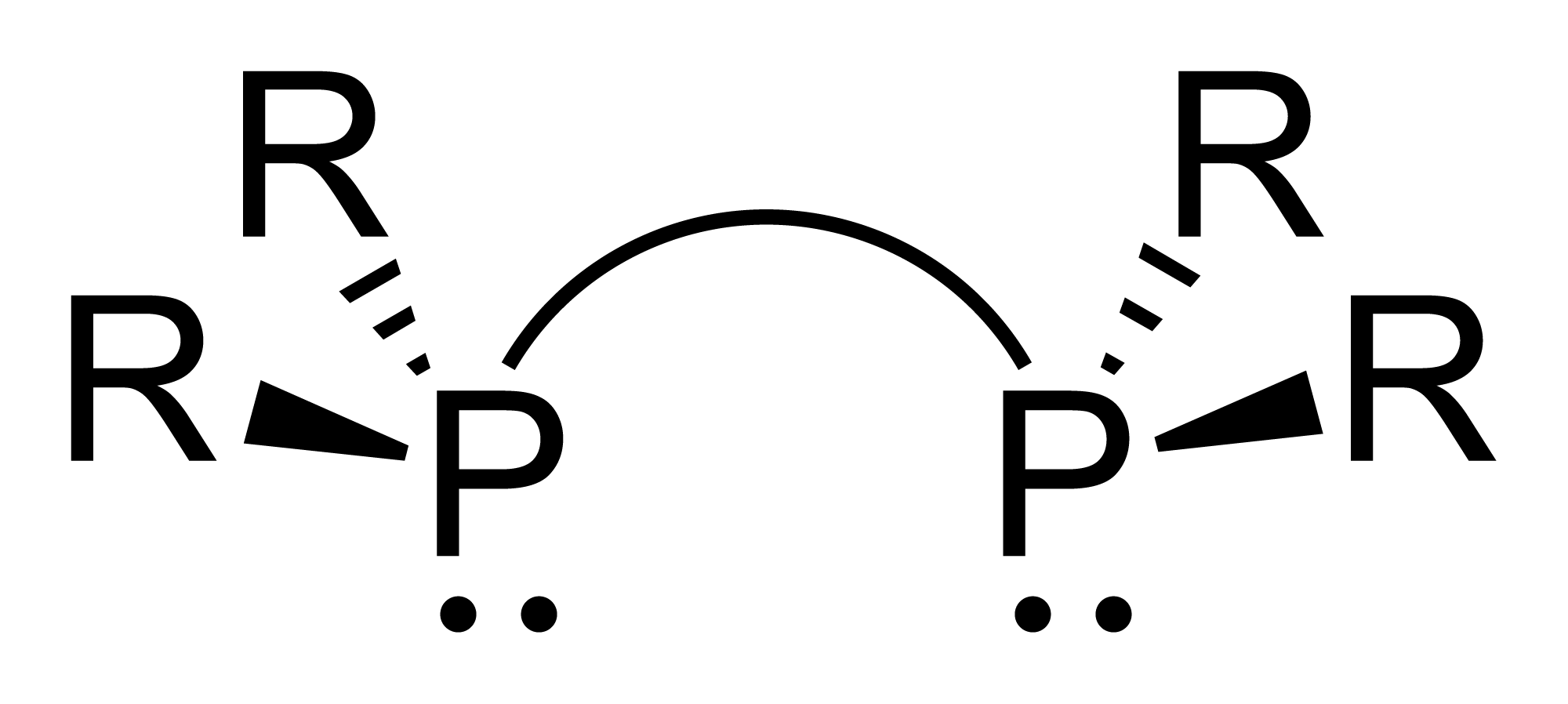
Dmpe
1,2-Bis(dimethylphosphino)ethane (dmpe) is a diphosphine ligand in coordination chemistry. It is a colorless, air-sensitive liquid that is soluble in organic solvents. With the formula (CHPMe), dmpe is used as a compact strongly basic spectator ligand (Me = methyl), Representative complexes include V(dmpe)(BH), Mn(dmpe)(AlH), Tc(dmpe)(CO)Cl, and Ni(dmpe)Cl. Synthesis It is synthesised by the reaction of methylmagnesium iodide with 1,2-bis(dichlorophosphino)ethane: :ClPCHCHPCl + 4 MeMgI → MePCHCHPMe + 4 MgICl Alternatively it can be generated by alkylation of sodium dimethylphosphide. The synthesis of dmpe from thiophosphoryl chloride Thiophosphoryl chloride is an inorganic compound with the chemical formula .Spilling, C. D. "Thiophosphoryl Chloride" in Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, Weinheim, 2001 . Article Online Posting Date: April 15, 2001 ... has led to serious accidents and has been abandoned. Related ligands * Bis(dicyclohexyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligand, phosphine ligands in inorganic chemistry, inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelate, chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in Homogeneous catalysis, homogeneous catalysts. Synthesis image:IPr2PCl.png, 220px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphine Ligand
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ... ligands in inorganic chemistry, inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelate, chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in Homogeneous catalysis, homogeneous catalysts. Synthesis image:IPr2PCl.png, 220px, Chlorodiisopropylphosphine is a popular building block for the preparation of diph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Chemistry
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectator Ligand
In coordination chemistry, a spectator ligand is a ligand that does not participate in chemical reactions of the complex. Instead, spectator ligands (vs "actor ligands") occupy coordination sites. Spectator ligands tend to be of polydentate, such that the M-spectator ensemble is inert kinetically. Although they do not participate in reactions of the metal, spectator ligands influence the reactivity of the metal center to which they are bound. These ligands are sometimes referred to as ancillary ligands. Several different classes of ligand exist that can be considered spectator ligands. A few examples include trispyrazolylborates (Tp), cyclopentadienyl ligands (Cp), and many chelating diphosphines such as 1,2-bis(diphenylphosphino)ethane ligands (dppe). Varying the substituents on the spectator ligands greatly influences the solubility, stability, electronic, and steric properties of the metal complex. In the area of platinum-based antineoplastic Platinum-based antineoplastic drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmagnesium Iodide
Methylmagnesium chloride is an organometallic compound with the general formula . This highly flammable, colorless, and moisture sensitive compound is the simplest Grignard reagent and is commercially available, usually as a solution in tetrahydrofuran. Synthesis and reactions Relative to the more commonly encountered methylmagnesium bromide and methylmagnesium iodide, methylmagnesium chloride offers the advantages of low equivalent weight and low cost. It is prepared by the reaction of methyl chloride and magnesium in ethyl ether. image:Methylmagnesium-chloride-THF-3D-balls.png, left, Structure of , which is representative of the species in donor solvents. As with most Grignard reagents, methylmagnesium chloride is highly solvated by ether solvents via Coordinate covalent bond, coordination from two oxygen atoms to give a tetrahedral molecular geometry, tetrahedrally bonded magnesium center. Like methyllithium, it is the synthetic equivalent to the methyl carbanion synthon. It r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(dichlorophosphino)ethane
1,2-Bis(dichlorophosphino)ethane is an organophosphorus compound with the formula (CH2PCl2)2. This colorless liquid is a precursor to chelating diphosphines. Synthesis and reactions It is prepared by the reaction of ethylene, white phosphorus, and phosphorus trichloride: :3 C2H4 + 0.5 P4 + 4 PCl3 → 3 (CH2PCl2)2 The compound reacts with Grignard reagents and secondary amines to give chelating ligands. An often practiced use of this compound is the synthesis of 1,2-bis(dimethylphosphino)ethane 1,2-Bis(dimethylphosphino)ethane (dmpe) is a diphosphine ligand in coordination chemistry. It is a colorless, air-sensitive liquid that is soluble in organic solvents. With the formula (CHPMe), dmpe is used as a compact strongly basic spectator .... Related compounds * 1,2-Bis(dichlorophosphino)benzene References {{DEFAULTSORT:Bis(dichlorophosphino)ethane, 1,2- Phosphines 1,2-Ethanediyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Organometallic Chemistry
The ''Journal of Organometallic Chemistry'' is a peer-reviewed scientific journal published by Elsevier, covering research on organometallic chemistry. According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 2.345. References External links * Organic chemistry journals Elsevier academic journals Academic journals established in 1964 English-language journals Monthly journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophosphoryl Chloride
Thiophosphoryl chloride is an inorganic compound with the chemical formula .Spilling, C. D. "Thiophosphoryl Chloride" in Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, Weinheim, 2001 . Article Online Posting Date: April 15, 2001 It is a colorless pungent smelling liquid that fumes in air. It is synthesized from phosphorus chloride and used to thiophosphorylate organic compounds, such as to produce insecticides. Synthesis Thiophosphoryl chloride can be generated by several reactions starting from phosphorus trichloride. The most common and practical synthesis, hence used in industrial manufacturing, is directly reacting phosphorus trichloride with excess sulfur at 180 °C. : Using this method, yields can be very high after purification by distillation. Catalysts facilitate the reaction at lower temperatures, but are not usually necessary. Alternatively, it is obtained by combining phosphorus pentasulfide and phosphorus pentachloride.Martin, D. R.; Duvall, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(dicyclohexylphosphino)ethane
Bis(dicyclohexylphosphino)ethane, abbreviated dcpe, is an organophosphorus compound with the formula (C6H11)2PCH2CH2P(C6H11)2. It is a white solid that is soluble in nonpolar organic solvents. The compound is used as a bulky and highly basic diphosphine ligand in coordination chemistry A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ....Sowa, J. R., Jr., Zanotti, V., Facchin, G., Angelici, R. J., "Calorimetric studies of the heats of protonation of the metal in Fe(CO)3(bidentate phosphine, arsine) complexes: effects of chelate ligands on metal basicity", J. Am. Chem. Soc. 1992, 114, 160. References {{DEFAULTSORT:Bis(dicyclohexylphosphino)ethane, 1,2- Chelating agents Diphosphines 1,2-Ethanediyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(diphenylphosphino)ethane
1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (PhPCH) (Ph = phenyl). It is a common symmetrical bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents. Preparation The preparation of dppe entails the alkylation of NaP(C6H5)2 with 1,2-dichloroethane: : Reactions The reduction of dppe by lithium give the disecondary phosphine: : Hydrolysis gives the bis(secondary phosphine). : : Treatment of dppe with hydrogen peroxide produces the phosphine oxides .Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons, Ltd Selective mono-oxidation of dppe can be achieved by benzylation followed by hydrolysis: : : Hydrogenation of dppe gives the ligand bis(dicyclohexylphosphino)ethane. Coordination complexes Many coordination complexes of dppe are known, and some are used as homogeneous catalysts. Dppe is almost invariably chelating, although there are examples of monodentate (e.g., W(CO)(d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |