|
Dineutron
Neutronium (or neutrium, neutrite, or element zero) is a hypothetical substance made purely of neutrons. The word was coined by scientist Andreas von Antropoff in 1926 (before the 1932 discovery of the neutron) for the hypothetical "element of atomic number zero" (with no protons in its nucleus) that he placed at the head of the periodic table (denoted by -). However, the meaning of the term has changed over time, and from the last half of the 20th century onward it has been also used to refer to extremely dense substances resembling the neutron-degenerate matter theorized to exist in the cores of neutron stars. In neutron stars Neutronium is used in popular physics literature to refer to the material present in the cores of neutron stars (stars which are too massive to be supported by electron degeneracy pressure and which collapse into a denser phase of matter). In scientific literature the term "neutron-degenerate matter" or simply neutron matter is used for this materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nuclear fission in 1938, the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity (nuclear test), Trinity, 1945). Neutrons are found, together with a similar number of protons in the atomic nucleus, nuclei of atoms. Atoms of a chemical element that differ only in neutron number are called isotopes. Free neutrons are produced copiously in nuclear fission and nuclear fusion, fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes. Neutron stars, formed from massive collapsing stars, consist of neutrons at the density of atomic nuclei but a total mass more than the Sun. Neutron properties and interactions ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Star Cross Section
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The neutron was discovered by James Chadwick in 1932, leading to the discovery of nuclear fission in 1938, the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945). Neutrons are found, together with a similar number of protons in the nuclei of atoms. Atoms of a chemical element that differ only in neutron number are called isotopes. Free neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes. Neutron stars, formed from massive collapsing stars, consist of neutrons at the density of atomic nuclei but a total mass more than the Sun. Neutron properties and interactions are described by nuclear physics. Neutrons are not elementary particles; each is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles Janet
Charles Janet (; 15 June 1849 – 7 February 1932) was a French engineer, company director, inventor and biologist. He is also known for his left-step periodic table of chemical elements. Life and work Janet graduated from the École Centrale Paris in 1872, and worked for some years as a chemist and engineer in a few factories in Puteaux (1872), Rouen (1873–74), and Saint-Ouen-sur-Seine, Saint-Ouen (1875–76). He was then employed by Philippe Alphonse Dupont, at Société A. Dupont & Cie, a factory that produced bone buttons and fine brushes. He married Berthe Marie Antonia Dupont, the daughter of the owner, in November 1877, and worked there for the rest of his life, finding time for research in various branches of science. Janet's collection of 50,000 fossils and other specimens was dispersed after his death. His studies of the morphology of the heads of ants, wasps and bees, and his micrographs were of remarkable quality. He also worked on plant biology and wrote a series o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraneutron
The tetraneutron is considered an unbound isotope with a lifetime around 10−22 seconds. The stability of this cluster of four neutrons is not supported by current models of nuclear forces. Recent empirical evidence is "consistent with a quasi-bound tetraneutron state existing for a very short time". Marqués' experiment Francisco-Miguel Marqués and co-workers at the GANIL accelerator in Caen used a particle accelerator to fire atomic nuclei at carbon targets and observed the "spray" of particles from the resulting collisions. In this case the experiment involved firing beryllium-14, boron-15 and lithium-11 nuclei at a small carbon target, the most successful being beryllium-14. This isotope of beryllium has a nuclear halo that consists of four clustered neutrons; this allows it to be easily separated intact in the high-speed collision with the carbon target. Current nuclear models suggest that four separate neutrons should result when beryllium-10 is produced, but the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
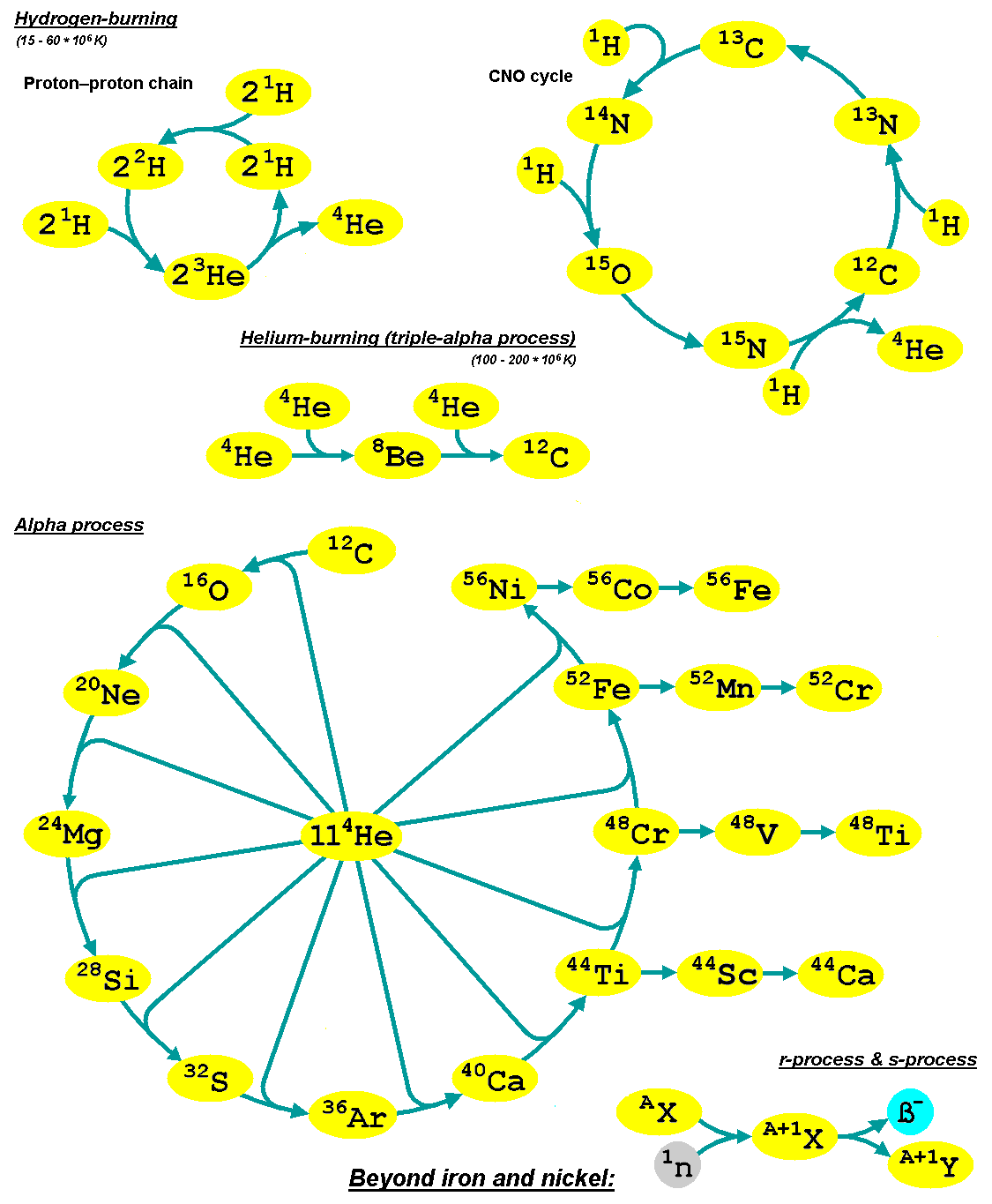
Abundance Of The Chemical Elements
The abundance of the chemical elements is a measure of the Type–token distinction#Occurrences, occurrences of the chemical elements relative to all other elements in a given environment. Abundance is measured in one of three ways: by mass fraction (chemistry), ''mass fraction'' (in commercial contexts often called ''weight fraction''), by ''mole fraction'' (fraction of atoms by numerical count, or sometimes fraction of molecules in gases), or by ''volume fraction''. Volume fraction is a common abundance measure in mixed gases such as planetary atmospheres, and is similar in value to molecular mole fraction for gas mixtures at relatively low densities and pressures, and ideal gas mixtures. Most abundance values in this article are given as mass fractions. The abundance of chemical elements in the universe is dominated by the large amounts of hydrogen and helium which were produced during Big Bang nucleosynthesis. Remaining elements, making up only about 2% of the universe, were lar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang nucleosynthesis. After about 20 minutes, the universe had expanded and cooled to a point at which these high-energy collisions among nucleons ended, so only the fastest and simplest reactions occurred, leaving our universe containing hydrogen and helium. The rest is traces of other elements such as lithium and the hydrogen isotope deuterium. Nucleosynthesis in stars and their explosions later produced the variety of elements and isotopes that we have today, in a process called cosmic chemical evolution. The amounts of total mass in elements heavier than hydrogen and helium (called 'metals' by astrophysicists) remains small (few percent), so that the universe still has approximately the same composition. Stars ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Review D
Physical may refer to: *Physical examination In a physical examination, medical examination, clinical examination, or medical checkup, a medical practitioner examines a patient for any possible medical signs or symptoms of a Disease, medical condition. It generally consists of a series of ..., a regular overall check-up with a doctor * ''Physical'' (Olivia Newton-John album), 1981 ** "Physical" (Olivia Newton-John song) * ''Physical'' (Gabe Gurnsey album) * "Physical" (Alcazar song) (2004) * "Physical" (Enrique Iglesias song) (2014) * "Physical" (Dua Lipa song) (2020) *"Physical (You're So)", a 1980 song by Adam & the Ants, the B side to " Dog Eat Dog" * ''Physical'' (TV series), an American television series *'' Physical: 100'', a Korean reality show on Netflix See also {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics Reports
''Physics Reports'' is a peer-reviewed scientific journal, a review section of '' Physics Letters'' that has been published by Elsevier since 1971. The journal publishes long and deep reviews on all aspects of physics. In average, the length of these reports is the same of a short book. These reports aim to make their main points intelligible to non-specialists. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 25.6, as reported in the official website of the Journal. References External links * Physics review journals Elsevier academic journals English-language journals Academic journals established in 1971 Weekly journals {{physics-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exotic Nuclei
A hypernucleus is similar to a conventional atomic nucleus, but contains at least one hyperon in addition to the normal protons and neutrons. Hyperons are a category of baryon particles that carry non-zero strangeness quantum number, which is conserved by the strong and electromagnetic interactions. A variety of reactions give access to depositing one or more units of strangeness in a nucleus. Hypernuclei containing the lightest hyperon, the lambda (Λ), tend to be more tightly bound than normal nuclei, though they can decay via the weak force with a mean lifetime of around . Sigma (Σ) hypernuclei have been sought, as have doubly-strange nuclei containing xi baryons (Ξ) or two Λ's. Nomenclature Hypernuclei are named in terms of their atomic number and baryon number, as in normal nuclei, plus the hyperon(s) which are listed in a left subscript of the symbol, with the caveat that atomic number is interpreted as the total charge of the hypernucleus, including charged hyperons suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Review Letters
''Physical Review Letters'' (''PRL''), established in 1958, is a peer-reviewed, scientific journal that is published 52 times per year by the American Physical Society. The journal is considered one of the most prestigious in the field of physics. Over a quarter of Physics Nobel Prize-winning papers between 1995 and 2017 were published in it. ''PRL'' is published both online and as a print journal. Its focus is on short articles ("letters") intended for quick publication. The Lead Editor is Hugues Chaté. The Managing Editor is Robert Garisto. History The journal was created in 1958. Samuel Goudsmit, who was then the editor of '' Physical Review'', the American Physical Society's flagship journal, organized and published ''Letters to the Editor of Physical Review'' into a new standalone journal'','' which became ''Physical Review Letters''. It was the first journal intended for the rapid publication of short articles, a format that eventually became popular in many other fiel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |