|
Dichloroisoprenaline
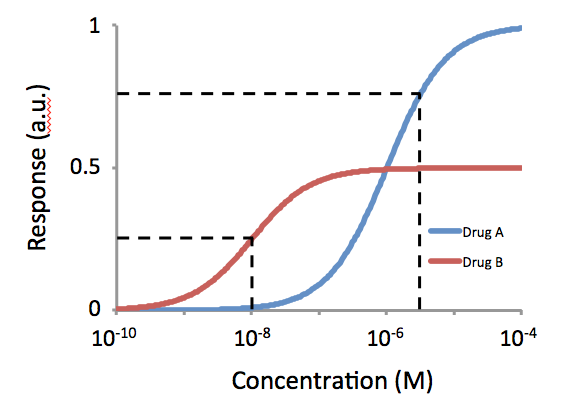
Dichloroisoprenaline (DCI), also known as dichloroisoproterenol, was the first beta blocker ever to be developed. It is non-selective for the β1-adrenergic and β2-adrenergic receptors. DCI has low potency and acts as a partial agonist/antagonist at these receptors. Although DCI was of no clinical value itself, further developments from DCI eventually led to the development of the clinical candidate pronethalol (withdrawn due to carcinogenicity) and subsequently propranolol (the first clinically successful beta blocker). Dichloroisoprenaline is a racemic mixture of enantiomer In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...s. References Beta blockers Chloroarenes Phenylethanolamines {{alkanederivative-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Blocker
Beta blockers, also spelled β-blockers, are a class of medications that are predominantly used to manage abnormal heart rhythms ( arrhythmia), and to protect the heart from a second heart attack after a first heart attack ( secondary prevention). They are also widely used to treat high blood pressure, although they are no longer the first choice for initial treatment of most people. Beta blockers are competitive antagonists that block the receptor sites for the endogenous catecholamines epinephrine (adrenaline) and norepinephrine (noradrenaline) on adrenergic beta receptors, of the sympathetic nervous system, which mediates the fight-or-flight response. Beta-adrenergic receptors are found on cells of the heart muscles, smooth muscles, airways, arteries, kidneys, and other tissues that are part of the sympathetic nervous system and lead to stress responses, especially when they are stimulated by epinephrine (adrenaline). Beta blockers interfere with the binding to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β1-adrenergic
The beta-1 adrenergic receptor (β1 adrenoceptor), also known as ADRB1, can refer to either the protein-encoding gene (gene ADRB1) or one of the four adrenergic receptors. It is a G-protein coupled receptor associated with the Gs heterotrimeric G-protein that is expressed predominantly in cardiac tissue. In addition to cardiac tissue, beta-1 adrenergic receptors are also expressed in the cerebral cortex. Historical Context W. B. Cannon postulated that there were two chemical transmitters or sympathins while studying the sympathetic nervous system in 1933. These E and I sympathins were involved with excitatory and inhibitory responses. In 1948, Raymond Ahlquist published a manuscript in the ''American Journal of Physiology'' establishing the idea of adrenaline having distinct actions on both alpha and beta receptors. Shortly afterward, Eli Lilly Laboratories synthesized the first beta-blocker, dichloroisoproterenol. General Information Structure ADRB-1 is a transmembrane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β2-adrenergic
The beta-2 adrenergic receptor (β2 adrenoreceptor), also known as ADRB2, is a cell membrane-spanning beta-adrenergic receptor that binds epinephrine (adrenaline), a hormone and neurotransmitter whose signaling, via adenylate cyclase stimulation through trimeric Gs proteins, increases cAMP, and, via downstream L-type calcium channel interaction, mediates physiologic responses such as smooth muscle relaxation and bronchodilation. Robert Lefkowitz and Brian Kobilka's study of the beta-2 adrenergic receptor as a model system earned them the 2012 Nobel Prize in Chemistry "for studies of G-protein-coupled receptors". The official symbol for the human gene encoding the β2 adrenoreceptor is ''ADRB2''. Gene The gene is intronless. Different polymorphic forms, point mutations, and/or downregulation of this gene are associated with nocturnal asthma, obesity and type 2 diabetes. Structure The 3D crystallographic structure (see figure and links to the right) of the β2-adrenergic rec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potency (pharmacology)
In pharmacology, potency or biological potency is a measure of a drug's biological activity expressed in terms of the dose required to produce a pharmacological effect of given intensity. A highly potent drug (e.g., fentanyl, clonazepam, risperidone, benperidol, bumetanide) evokes a given response at low concentrations, while a drug of lower potency (e.g. morphine, alprazolam, ziprasidone, haloperidol, furosemide) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness nor more side effects nor less side effects. Types of potency The International Union of Basic and Clinical Pharmacology (IUPHAR) has stated that "potency is an imprecise term that should always be further defined", and lists of types of potency as follows: Miscellaneous Lysergic acid diethylamide (LSD) is one of the most potent psychoactive drug A psychoactive drug, psychopharmaceutical, mind-altering drug, consciousness-altering drug, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Agonist
In pharmacology, partial agonists are drugs that bind to and activate a given Receptor (biochemistry), receptor, but have only partial Intrinsic activity, efficacy at the receptor relative to a full agonist. They may also be considered Ligand (biochemistry), ligands which display both agonistic and Receptor antagonist, antagonistic effects—when both a full agonist and partial agonist are present, the partial agonist actually acts as a competitive antagonist, competing with the full agonist for receptor occupancy and producing a net decrease in the receptor activation observed with the full agonist alone. Clinically, partial agonists can be used to activate receptors to give a desired submaximal response when inadequate amounts of the endogenous ligand are present, or they can reduce the overstimulation of receptors when excess amounts of the endogenous ligand are present. Some currently common drugs that have been classed as partial agonists at particular receptors include buspi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
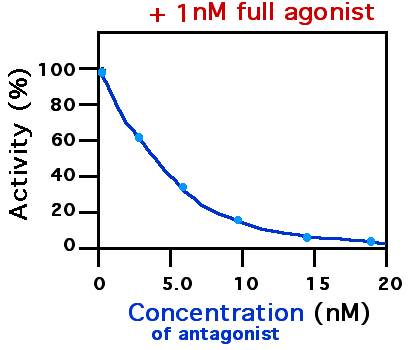
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves ." '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and Signal_transduction, transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and produce physiological responses, such as a change in the electrophysiology, electrical activity of a cell. For example, GABA, an inhibitory neurotransmitter, inhibits electrical activity of neurons by binding to GABAA receptor, GABA receptors. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand (biochemistry), ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Cell surface receptors, also known as transmembrane receptors, include ligand-gated ion channels, G prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pronethalol
Pronethalol (also known as nethalide or compound 38,174; trade name Alderlin) was an early non-selective beta blocker clinical candidate. It was the first beta blocker to be developed by James Black and associates at Imperial Chemical Industries, and the first to enter clinical use, in November 1963. However, it was never used widely due to carcinogenicity in mice, which was thought to result from formation of a carcinogenic naphthalene epoxide metabolite, and was superseded by propranolol from 1965 onward. See also * Beta blocker Beta blockers, also spelled β-blockers, are a class of medications that are predominantly used to manage abnormal heart rhythms ( arrhythmia), and to protect the heart from a second heart attack after a first heart attack ( secondary prevention ... * Discovery and development of beta-blockers * Naphthylaminopropane References Beta blockers 2-Naphthyl compounds Naphthylethylamines Phenylethanolamines {{antihypertensive-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogenicity
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and Biological agent, biologic agents such as viruses and carcinogenic bacteria, bacteria. Most carcinogens act by creating mutations in DNA that disrupt a cell's normal processes for regulating growth, leading to uncontrolled cellular proliferation. This occurs when the cell's DNA repair processes fail to identify DNA damage allowing the defect to be passed down to daughter cells. The damage accumulates over time. This is typically a multi-step process during which the regulatory mechanisms within the cell are gradually dismantled allowing for unchecked Cell division, cellular division. The specific mechanisms for carcinogenic activity is unique to each agent and cell type. Carcinogens can be broadly categorized, however, as activation-dependent and activation-independent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propranolol
Propranolol is a medication of the beta blocker class. It is used to treat hypertension, high blood pressure, some types of cardiac dysrhythmia, irregular heart rate, thyrotoxicosis, capillary hemangiomas, akathisia, performance anxiety, and essential tremors, as well to prevent migraine headaches, and to prevent further heart problems in those with angina or previous myocardial infarction, heart attacks. It can be taken oral administration, orally or by intravenous injection. The formulation that is taken orally comes in short-acting and long-acting versions. Propranolol appears in the blood after 30 minutes and has a maximum effect between 60 and 90 minutes when taken orally. Common side effects include nausea, abdominal pain, and constipation. It may worsen the symptoms of asthma. Propranolol may cause teratogen, harmful effects for the baby if taken during pregnancy; however, its use during breastfeeding is generally considered to be safe. It is a non-selective beta block ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemic Mixture
In chemistry, a racemic mixture or racemate () is a mixture that has equal amounts (50:50) of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology The word ''racemic'' derives from Latin , meaning pertaining to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities which are mirror images of each other and non-superposable. Enantiomer molecules are like right and left hands: one cannot be superposed onto the other without first being converted to its mirror image. It is solely a relationship of chirality (chemistry), chirality and the permanent three-dimensional relationships among molecules or other chemical structures: no amount of re-orientation of a molecule as a whole or conformational isomerism, conformational change converts one chemical into its enantiomer. Chemical structures with chirality rotate plane-polarized light. A mixture of equal amounts of each enantiomer, a ''racemic mixture'' or a ''racemate'', does not rotate light. Stereoisomers include both enantiomers and diastereomers. Diaste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |