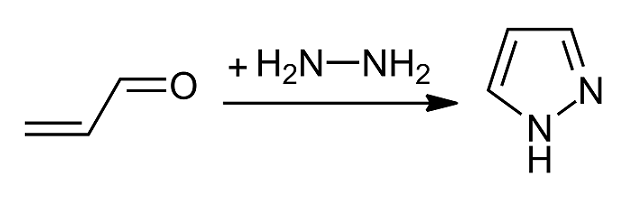|
Dibenzoylmethane
Dibenzoylmethane (DBM) is an organic compound with the formula (C6H5C(O))2CH2. DBM is the name for a 1,3-diketone, but the compound exists primarily as one of two equivalent enol tautomers. DBM is a white solid. Due UV-absorbing properties, derivatives of DBM such as avobenzone, have found applications as sunscreen products. Synthesis and reactions DBM is prepared by condensation of ethyl benzoate with acetophenone. Like other 1,3-diketones (or their enols), DBM condenses with a variety of bifunctional reagents to give heterocycles. Hydrazine gives diphenylpyrazole. Urea and thiourea also condense to give six-membered rings. With metal salts, the conjugate base of DBM forms complexes akin to the metal acetylacetonates. Occurrence and medicinal properties left, Curcumin, structurally related to DBM, is the bright yellow component of the spice turmeric. Dibenzoylmethane (DBM) is a minor constituent in the root extract of Licorice (''Glycyrrhiza glabra'' in the family Legumin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Avobenzone
Avobenzone (trade names Parsol 1789, Milestab 1789, Eusolex 9020, Escalol 517, Neo Heliopan 357 and others, International Nomenclature of Cosmetic Ingredients, INCI Butyl Methoxydibenzoylmethane) is an organic molecule and an oil-soluble ingredient used in sunscreen products to absorb the full spectrum of Ultraviolet, UVA rays. History Avobenzone was patented in 1973 and was approved in the EU in 1978. It was approved by the Food and Drug Administration, FDA in 1988. As of 2021, the FDA announced that they do not support avobenzone as being generally recognized as safe and effective (GRASE) citing the need for additional safety data. Avobenzone was banned in 2020 by the Palau government citing reef-toxicity concerns. Properties Pure avobenzone is a whitish to yellowish crystalline powder with a weak odor, dissolving in isopropanol, dimethyl sulfoxide, decyl oleate, capric acid/caprylic, triglycerides and other oils. It is not soluble in water. Avobenzone is a dibenzoylmethane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Curcumin
Curcumin is a bright yellow chemical produced by plants of the ''Curcuma longa'' species. It is the principal curcuminoid of turmeric (''Curcuma longa''), a member of the ginger family, Zingiberaceae. It is sold as a herbal supplement, cosmetics ingredient, food flavoring, and food coloring. Chemically, curcumin is a polyphenol, more particularly a diarylheptanoid, belonging to the group of curcuminoids, which are natural phenol, phenolic pigments responsible for the yellow color of turmeric. Laboratory and clinical research have not confirmed any medical use for curcumin. It is difficult to study because it is both unstable and poorly bioavailable. It is unlikely to produce useful leads for drug development as a lead compound. History Curcumin was named in 1815 when Henri Auguste Vogel and Pierre Joseph Pelletier reported the first isolation of a "yellow coloring-matter" from the rhizomes of turmeric. Later, it was found to be a mixture of resin and turmeric oil. In 1910, Mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelating Agents
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity. The word ''chelation'' is derived from Greek χηλή, ''chēlē'', meaning "claw"; the ligands lie around the central atom like the claws of a crab. The term ''chelate'' () was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or ''chele'' (Greek) of the crab or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings." Chelation is useful in applications such as providing nutritional supplements, in chela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Ketones
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfaction, olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of Resonance (chemistry), resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-covalent bond, bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Friedrich August Kekulé ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoylacetone
Benzoylacetone is the organic compound with the nominal formula C6H5C(O)CH2C(O)CH3. As a 1,3-dicarbonyl, it is a precursor to many heterocycles, such as pyrazoles. It exists predominantly as the enol In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ... tautomer C6H5C(OH)=CHC(O)CH3. Its conjugate base (pKa=8.7) forms stable complexes with transition metals and lanthanides.{{cite journal , doi=10.1002/(SICI)1521-4095(199911)11:163.0.CO;2-W, title=Narrow Bandwidth Luminescence from Blends with Energy Transfer from Semiconducting Conjugated Polymers to Europium Complexes, year=1999, last1=McGehee, first1=M. D., last2=Bergstedt, first2=T., last3=Zhang, first3=C., last4=Saab, first4=A. P., last5=o'Regan, first5=M. B., last6=Bazan, first6=G. C., last7=Srdanov, first7=V. I., last8=Heeger, fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trazodone
Trazodone is an antidepressant medication used to treat major depressive disorder, anxiety disorders, and insomnia. It is a phenylpiperazine compound of the serotonin antagonist and reuptake inhibitor (SARI) class. The medication is taken orally. Common side effects include dry mouth, feeling faint, vomiting, and headache. More serious side effects may include suicide, mania, irregular heart rate, and pathologically prolonged erections. It is unclear if use during pregnancy or breastfeeding is safe. Trazodone also has sedating effects. Trazodone was approved for medical use in the United States in 1981. It is available as a generic medication. In 2022, it was the eighteenth most commonly prescribed medication in the United States, with more than 27million prescriptions. Medical uses Depression The primary use of trazodone is the treatment of unipolar major depression with or without anxiety. Data from open and double-blind trials suggest that the antidepressant eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Curcumin
Curcumin is a bright yellow chemical produced by plants of the ''Curcuma longa'' species. It is the principal curcuminoid of turmeric (''Curcuma longa''), a member of the ginger family, Zingiberaceae. It is sold as a herbal supplement, cosmetics ingredient, food flavoring, and food coloring. Chemically, curcumin is a polyphenol, more particularly a diarylheptanoid, belonging to the group of curcuminoids, which are natural phenol, phenolic pigments responsible for the yellow color of turmeric. Laboratory and clinical research have not confirmed any medical use for curcumin. It is difficult to study because it is both unstable and poorly bioavailable. It is unlikely to produce useful leads for drug development as a lead compound. History Curcumin was named in 1815 when Henri Auguste Vogel and Pierre Joseph Pelletier reported the first isolation of a "yellow coloring-matter" from the rhizomes of turmeric. Later, it was found to be a mixture of resin and turmeric oil. In 1910, Mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Licorice
Liquorice (Commonwealth English) or licorice (American English; see spelling differences; ) is the common name of ''Glycyrrhiza glabra'', a flowering plant of the bean family Fabaceae, from the root of which a sweet, aromatic flavouring is extracted. The liquorice plant is an herbaceous perennial legume native to West Asia, North Africa, and Southern Europe. Liquorice is used as a flavouring in confectionery, tobacco, beverages, and pharmaceuticals, and is marketed as a dietary supplement. Liquorice extracts have been used in herbalism and traditional medicine. Excessive consumption of liquorice (more than per day of pure glycyrrhizinic acid, a key component of liquorice) can lead to undesirable consequences. Clinically, it is suspected that overindulgence in liquorice may manifest as unexplained hypertension, low blood potassium levels ( hypokalemia), and muscle weakness in individuals. Consuming liquorice should be avoided during pregnancy. Etymology The word ''liquo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Acetylacetonates
Metal acetylacetonates are coordination complexes derived from the acetylacetone, acetylacetonate anion () and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCO−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical (dialdehydes, diketones, diesters, ''etc.'') or unsymmetrical (keto-esters, keto-acids, ''etc.''). 1,2-Dicarbonyls 1,2-Dialdehyde The only 1,2-dialdehyde is glyoxal, . Like many alkyldialdehydes, glyoxal is encountered almost exclusively as its hydrate and oligomers thereof. These derivatives often behave equivalently to the aldehydes since hydration is reversible. Glyoxal condenses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazole
Pyrazole is an organic compound with the chemical formula, formula . It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in Arene substitution pattern, ortho-substitution. Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Properties Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). According to X-ray crystallography, the compound is planar. The two C-N distances are similar, both near 1.33 Å History The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883. In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |