|
Deltorphin II
Deltorphin, also known as deltorphin A and dermenkephalin, is a naturally occurring, exogenous opioid heptapeptide and thus, exorphin, with the amino acid sequence Tyr-D-Met-Phe-His-Leu-Met-Asp-NH2. Along with the other deltorphins (such as deltorphin I and deltorphin II) and the dermorphins, deltorphin is endogenous to frogs of the genus ''Phyllomedusa'' such as '' P. bicolor'' and '' P. sauvagei'' where it is produced in their skin, and is not known to occur naturally in any other species. Deltorphin is one of the highest affinity and most selective naturally occurring opioid peptides known, acting as a very potent and highly specific agonist of the δ-opioid receptor. Deltorphins have an unusually high blood–brain barrier penetration rate. The nonselective opiate antagonist naloxone inhibits deltorphin uptake by brain microvessels, but neither the selective δ-opioid antagonist naltrindole nor a number of opioid peptides with different affinities for δ- or μ-opioid recep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis (both semisynthesis and total synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets). The term ''natural product'' has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients. Within the field of organic chemistry, the definition of natural products is usually restricted to organic compounds isolated from natural sources that are produced by the pathways of primary or secondary metabolism. Within the field of medicinal chemistry, the definition is often further restricted to secondary metabolites. Secondary metabolites (or specialized meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phyllomedusa Sauvagii
''Phyllomedusa sauvagii'', the waxy monkey leaf frog or waxy monkey tree frog, is a species of frog in the subfamily Phyllomedusinae. It is native to South America, where it occurs in Argentina, Bolivia, Paraguay and Brazil. This species is arboreal, living in the vegetation of the Gran Chaco. Reproduction and embryo development The waxy monkey leaf frog breeds during the rainy season, which typically lasts from October until March. Mating does not occur continuously throughout the season, only during or shortly after periods of heavy rainfall. Males will find a shrub or tree near or in a body of water where they will begin to vocalize to attract females. They are amplectant maters and, as such, breeding pairs create their nest of eggs together. The pair will move towards their oviposition site, usually on a leaf overhanging a body of water, where the male will assist the female in laying her eggs while simultaneously fertilizing them. A critical aspect of the reproductive suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dermorphin
Dermorphin is a hepta-peptide first isolated from the skin of South American frogs belonging to the genus ''Phyllomedusa''. The peptide is a natural opioid that binds as an agonist with high potency and selectivity to mu opioid receptors. Dermorphin is about 30–40 times more potent than morphine. The amino acid sequence of dermorphin is H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2. Dermorphin is not found in humans or other mammals and similar D-amino acid peptides have only been found in bacteria, amphibians, and molluscs. Dermorphin appears to be made in these through an unusual posttranslational modification carried out by an amino acid isomerase. This unusual process is needed because the D-alanine in this peptide is not among the 20 amino acids coded for in the genetic code and thus the peptide cannot be synthesized in the usual way from the encodings in the genome of an organism. Illicit use Dermorphin has been illegally used in horse racing as a performance-enhancing drug. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deltorphin I
Deltorphin I, also known as D-Ala2">small>D-Ala2eltorphin I or deltorphin C, is a naturally occurring, exogenous opioid heptapeptide and hence, exorphin, with the amino acid sequence Tyr-D-Ala-Phe-Asp-Val-Val-Gly-NH2. While not known to be endogenous to humans or other mammals, deltorphin I, along with the other deltorphins and the dermorphins, is produced naturally in the skin of species of ''Phyllomedusa'', a genus of frogs native to South and Central America. Deltorphin possesses very high affinity and selectivity as an agonist for the δ-opioid receptor, and on account of its unusually high blood-brain-barrier penetration rate, produces centrally-mediated analgesic effects in animals even when administered peripherally. See also * Deltorphin Deltorphin, also known as deltorphin A and dermenkephalin, is a naturally occurring, exogenous opioid heptapeptide and thus, exorphin, with the amino acid sequence Tyr-D-Met-Phe-His-Leu-Met-Asp-NH2. Along with the other deltorp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naltrindole
Naltrindole is a highly potent, highly selective delta opioid receptor antagonist used in biomedical research. In May 2012 a paper was published in ''Nature'' with the structure of naltrindole in complex with the mouse δ-opioid G-protein coupled receptor, solved by X-ray crystallography. Drug design Since peptide compounds are unable to cross the blood–brain barrier, researchers developed naltrindole to be a non-peptide antagonist analog of the delta-preferring endogenous opiate enkephalin. Enkephalin contains an aromatic phenyl group on its Phe4 residue, which was hypothesized to be the "address" sequence responsible for the opiate's delta opioid receptor affinity. Thus, attachment of a phenyl-containing indole molecule to the C-ring of naltrexone's morphinan Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Typical examples includ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naloxone
Naloxone, sold under the brand name Narcan among others, is an opioid antagonist, a medication used to reverse or reduce the effects of opioids. For example, it is used to restore breathing after an opioid overdose. Effects begin within two minutes when given intravenously, five minutes when injected into a muscle, and ten minutes as a nasal spray. Naloxone blocks the effects of opioids for 30 to 90 minutes. Administration to opioid-dependent individuals may cause symptoms of opioid withdrawal, including restlessness, agitation, nausea, vomiting, a fast heart rate, and sweating. To prevent this, small doses every few minutes can be given until the desired effect is reached. In those with previous heart disease or taking medications that negatively affect the heart, further heart problems have occurred. It appears to be safe in pregnancy, after having been given to a limited number of women. Naloxone is a non-selective and competitive opioid receptor antagonist. It revers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
δ-opioid Receptor
The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi alpha subunit, Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and Neocortex, neocortical regions of the brain. Function The endogenous system of opioid receptors is well known for its analgesic potential; however, the exact role of δ-opioid receptor activation in pain modulation is largely up for debate. This also depends on the model at hand since receptor activity is known to change from species to species. Activation of delta receptors produces analgesia, perhaps as significant potentiators of μ-opioid receptor agonists. However, it seems like delta agonism provides heavy poten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an Receptor antagonist, antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology The word originates from the Ancient Greek, Greek word (''agōnistēs''), "contestant; champion; rival" < (''agōn''), "contest, combat; exertion, struggle" < (''agō''), "I lead, lead towards, conduct; drive." Types of agonists Receptor (biochemistry), Receptors can be activated by either endogenous agonists (such as hormones and neurotransmitters) or exogenous agonists (such as medication, drugs), resulting in a biological response. A physiological agonism an ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
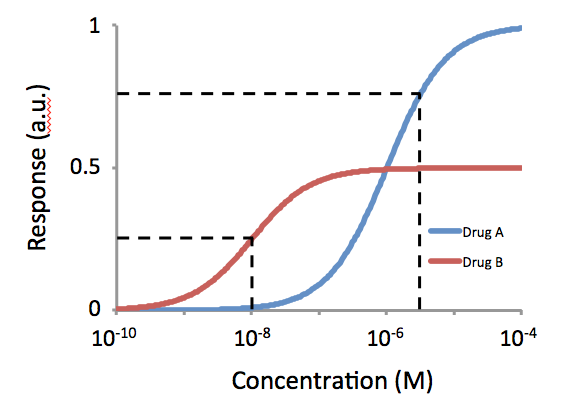
Potency (pharmacology)
In pharmacology, potency or biological potency is a measure of a drug's biological activity expressed in terms of the dose required to produce a pharmacological effect of given intensity. A highly potent drug (e.g., fentanyl, clonazepam, risperidone, benperidol, bumetanide) evokes a given response at low concentrations, while a drug of lower potency (e.g. morphine, alprazolam, ziprasidone, haloperidol, furosemide) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness nor more side effects nor less side effects. Types of potency The International Union of Basic and Clinical Pharmacology (IUPHAR) has stated that "potency is an imprecise term that should always be further defined", and lists of types of potency as follows: Miscellaneous Lysergic acid diethylamide (LSD) is one of the most potent psychoactive drug A psychoactive drug, psychopharmaceutical, mind-altering drug, consciousness-altering drug, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Selectivity
In chemistry, binding selectivity is defined with respect to the binding of ligands to a substrate forming a complex. Binding selectivity describes how a ligand may bind more preferentially to one receptor than another. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry and in chemical separation processes. Selectivity coefficient The concept of selectivity is used to quantify the extent to which one chemical substance, A, binds each of two other chemical substances, B and C. The simplest case is where the complexes formed have 1:1 stoichiometry. Then, the two interactions may be characterized by equilibrium constants and .The constant used here are ''association'' constants. ''Dissociation'' constants are used in some contexts. A dissociation constant is the reciprocal of an association constant. \begin \ce;& \quad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity (pharmacology)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phyllomedusa Bicolor
''Phyllomedusa bicolor'', the giant leaf frog, bicolor tree-frog, giant monkey frog, or waxy-monkey treefrog, is a species of leaf frog. It can be found in the Amazon basin of Brazil, Colombia ( Amazonas), Bolivia, and Peru, and can also be found in the Guianan Region of Venezuela and the Guianas, and in Cerrado of the state of Maranhão in Brazil. Description Males measure and females in snout–vent length. The dorsum is lime green whereas the belly is white to yellow-white or cream. Lower lips, chest and front legs bear sparse white spots with dark frames; these are more dense on the flanks and hind legs. Fingers are transparent brown and have large, green adhesive discs. There is a prominent gland extending from behind each eye over the tympanum. The iris is dark gray. Distribution and habitat It is found throughout the Amazon Rainforest in Bolivia, the Guianas, Brazil, Colombia, Venezuela, and Peru. This frog has been found in gallery forest. Ecology and behavi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |