|
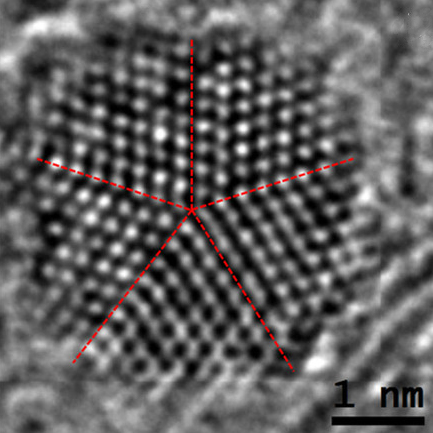
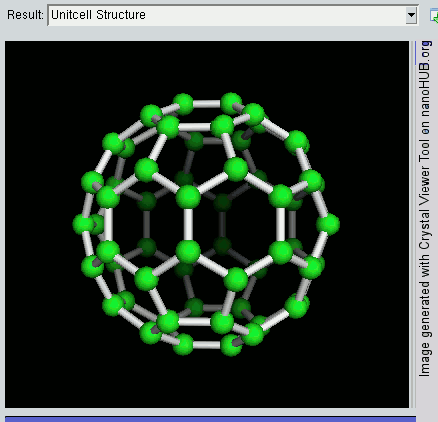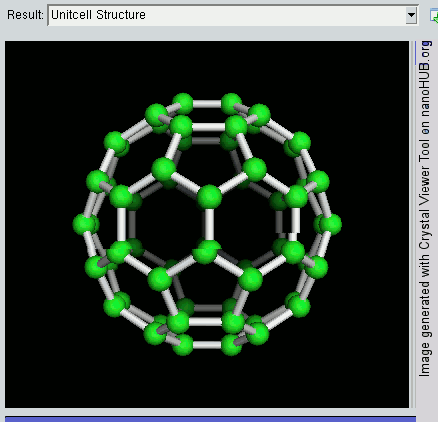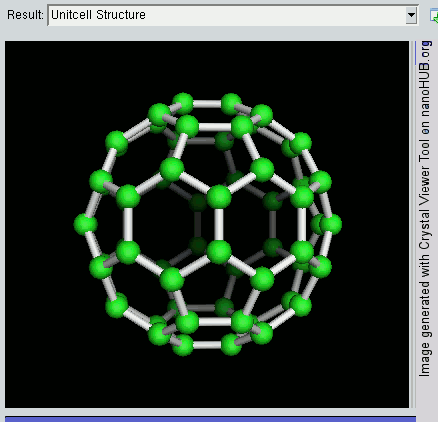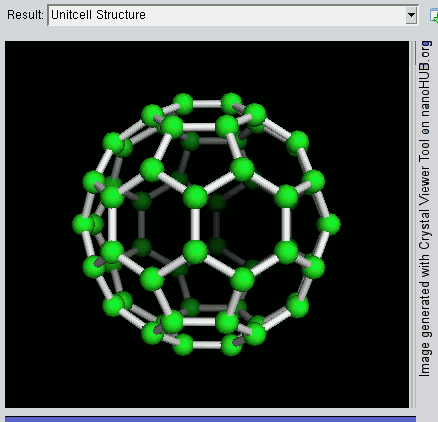
Decahedral Nanoparticle
A fiveling, also known as a decahedral nanoparticle, a multiply-twinned particle (MTP), a pentagonal nanoparticle, a pentatwin, or a five-fold twin is a type of twinned crystal that can exist at sizes ranging from nanometers to millimetres. It contains five different single crystals arranged around a common axis. In most cases each unit has a Cubic crystal system#Bravais lattice, face centered cubic (fcc) arrangement of the atoms, although they are also known for other types of crystal structure. They nucleate at quite small sizes in the nanometer range, but can be grown much larger. They have been found in mineral crystals excavated from mines such as pentagonite or native gold from Ukraine, in rods of metals grown via electrochemical processes and in nanoparticles produced by the condensation of metals either onto substrates or in inert gases. They have been investigated for their potential uses in areas such as improving the efficiency of solar cell or heterogeneous catalysis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanomaterials
Nanomaterials describe, in principle, chemical substances or materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale). Nanomaterials research takes a materials science-based approach to nanotechnology, leveraging advances in materials metrology and synthesis which have been developed in support of microfabrication research. Materials with structure at the nanoscale often have unique optical, electronic, thermo-physical or mechanical properties. Nanomaterials are slowly becoming commercialized and beginning to emerge as commodities. Definition In ISO/TS 80004, ''nanomaterial'' is defined as the "material with any external dimension in the nanoscale or having internal structure or surface structure in the nanoscale", with ''nanoscale'' defined as the "length range approximately from 1 nm to 100 nm". This includes both ''nano-objects'', which are discrete pieces of material, and ''nanostructu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Library Of Scotland
The National Library of Scotland (NLS; ; ) is one of Scotland's National Collections. It is one of the largest libraries in the United Kingdom. As well as a public programme of exhibitions, events, workshops, and tours, the National Library of Scotland has reading rooms where visitors can access the collections. It is the legal deposit library of Scotland and is a member of Research Libraries UK (RLUK) and the Consortium of European Research Libraries (CERL). There are over 24 million items held at the Library in various formats including books, annotated manuscripts and first-drafts, postcards, photographs, and newspapers. The library is also home to Scotland's Moving Image Archive, a collection of over 46,000 videos and films. Notable items amongst the collection include copies of the Gutenberg Bible, Charles Darwin's letter with which he submitted the manuscript of ''On the Origin of Species,'' the First Folio of Shakespeare, the Glenriddell Manuscripts, and the last ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macle
Macle is a term used in crystallography. It is a crystalline form, twin-crystal or double crystal (such as chiastolite). It is crystallographic twin according to the spinel twin law and is seen in octahedral crystals or minerals such as diamond and spinel. The twin law name comes from the fact that is commonly observed in the mineral spinel. A version with five units about a common axis is called a fiveling. ''Macle'' is an old French word, a heraldic term for a voided Lozenge (shape)">lozenge (one diamond Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ... shape within another). Etymologically the word is derived from the Latin ''macula'' meaning spot, mesh, or hole. Bibliography * Georges Friedel (1904) "Étude sur les groupements cristallins", ''Extrait du Bulletin de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Twinning
Crystal twinning occurs when two or more adjacent crystals of the same mineral are oriented so that they share some of the same crystal lattice points in a symmetrical manner. The result is an intergrowth of two separate crystals that are tightly bonded to each other. The surface along which the lattice points are shared in twinned crystals is called a composition surface or twin plane. Crystallographers classify twinned crystals by a number of twin laws, which are specific to the crystal structure. The type of twinning can be a diagnostic tool in mineral identification. There are three main types of twinning. The first is growth twinning which can occur both in very large and very small particles. The second is transformation twinning, where there is a change in the crystal structure. The third is deformation twinning, in which twinning develops in a crystal in response to a shear stress, and is an important mechanism for permanent shape changes in a crystal. Definition T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gustav Rose
Prof Gustavus ("Gustav") Rose Royal Society of London, FRSFor HFRSE (18 March 1798 – 15 July 1873) was a German mineralogist who was a native of Berlin. He was President of the German Geological Society from 1863 to 1873. Life He was born in Berlin the son of pharmacologist Valentin Rose (pharmacologist), Valentin Rose. Rose was a graduate of the University of Berlin, where he was a student of mineralogist Christian Samuel Weiss (1780–1856). He also studied under Swedish people, Swedish Physical chemistry, physical chemist Jöns Jakob Berzelius (1779–1848) in Stockholm. While studying with Berzelius, Rose met German chemist Eilhard Mitscherlich (1794–1863), with whom he maintained a lifelong friendship. Rose provided assistance to Mitscherlich's development of the law of Isomorphism (crystallography), isomorphism. In 1826 he became an associate professor of mineralogy in Berlin. In 1829, with German natural history, naturalists Alexander von Humboldt (1769–1859) and Chri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marcasite
The mineral marcasite, sometimes called "white iron pyrite", is iron sulfide (FeS2) with orthorhombic crystal structure. It is physically and crystallographically distinct from pyrite, which is iron sulfide with cubic crystal structure. Both structures contain the disulfide S22− ion, having a short bonding distance between the sulfur atoms. The structures differ in how these di-anions are arranged around the Fe2+ cations. Marcasite is lighter and more brittle than pyrite. Specimens of marcasite often crumble and break up due to the unstable crystal structure. On fresh surfaces, it is pale yellow to almost white and has a bright metallic luster. It tarnishes to a yellowish or brownish color and gives a black streak. It is a brittle material that cannot be scratched with a knife. The thin, flat, tabular crystals, when joined in groups, are called "cockscombs". In the late medieval and early modern eras, the word "marcasite" meant all iron sulfides in general, including b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system, in particular a chemical compound or a polymer. Colloquially, it may instead refer to kinetic persistence, the shelf-life of a metastable substance or system; that is, the timescale over which it begins to degrade. Thermodynamic stability occurs when a system is in its lowest energy state, or in chemical equilibrium with its environment. This may be a dynamic equilibrium in which individual atoms or molecules change form, but their overall number in a particular form is conserved. This type of chemical thermodynamic equilibrium will persist indefinitely unless the system is changed. Chemical systems might undergo changes in the phase of matter or a set of chemical reactions. State A is said to be more thermodynamically stable than state B if the Gibbs free energy of the change from A to B is positive. Versus reactivity Thermodynamic stability applies to a particular system. The reactivity of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemisorption
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like corrosion, and subtler effects associated with heterogeneous catalysis, where the catalyst and reactants are in different phases. The strong interaction between the adsorbate and the substrate surface creates new types of electronic bonds. In contrast with chemisorption is physisorption, which leaves the chemical species of the adsorbate and surface intact. It is conventionally accepted that the energetic threshold separating the binding energy of "physisorption" from that of "chemisorption" is about 0.5 eV per adsorbed species. Due to specificity, the nature of chemisorption can greatly differ, depending on the chemical identity and the surface structural properties. The bond between the adsorbate and adsorbent in chemisorption is e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disclination
In crystallography, a disclination is a line defect in which there is compensation of an angular gap. They were first discussed by Vito Volterra in 1907, who provided an analysis of the elastic strains of a wedge disclination. By analogy to dislocations in crystals, the term, ''disinclination'', was first used by Charles Frank (physicist), Charles Frank and since then has been modified to its current usage, ''disclination''. They have since been analyzed in some detail particularly by Roland deWit. Disclinations are characterized by an angular vector (called a Frank vector), and the line of the disclination. When the vector and the line are the same they are sometimes called ''wedge disclinations'' which are common in fiveling, decahedral nanoparticles. When the Frank vector and the line of the disclination are at right angles they are called ''twist disclinations''. As pointed out by John D. Eshelby there is an intricate connection between disclinations and dislocations, with disl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |