|
Cytochrome C Peroxidase
Cytochrome ''c'' peroxidase, or CCP, is a water-soluble heme-containing enzyme of the peroxidase family that takes reducing equivalents from cytochrome ''c'' and reduces hydrogen peroxide to water: :CCP + H2O2 + 2 ferrocytochrome ''c'' + 2H+ → CCP + 2H2O + 2 ferricytochrome ''c'' CCP can be derived from aerobically grown yeast strains and can be isolated in both native and recombinant forms with high yield from ''Saccharomyces cerevisiae.'' The enzyme’s primary function is to eliminate toxic radical molecules produced by the cell which are harmful to biological systems. It works to maintain low concentration levels of hydrogen peroxide, which is generated by the organism naturally through incomplete oxygen reduction. When glucose levels in fast growing yeast strains are exhausted, the cells turn to respiration which raises the concentration of mitochondrial H2O2. In addition to its peroxidase activity, it acts as a sensor and a signaling molecule to exogenous H2O2, which acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prosthetic group, component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 Vinyl group, vinyl and 2 propionic acid side chains. Heme is biosynthesized in both the bone marrow and the liver. Heme plays a critical role in multiple different redox reactions in mammals, due to its ability to carry the oxygen molecule. Reactions include oxidative metabolism (cytochrome c oxidase, succinate dehydrogenase), xenobiotic detoxification via cytochrome P450 pathways (including Drug metabolism, metabolism of some drugs), gas sensing (Guanylate cyclase, guanyl cyclases, nitric oxide synthase), and microRNA processing (DGCR8). Heme is a coordination complex "consisting of an iron ion coordinated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
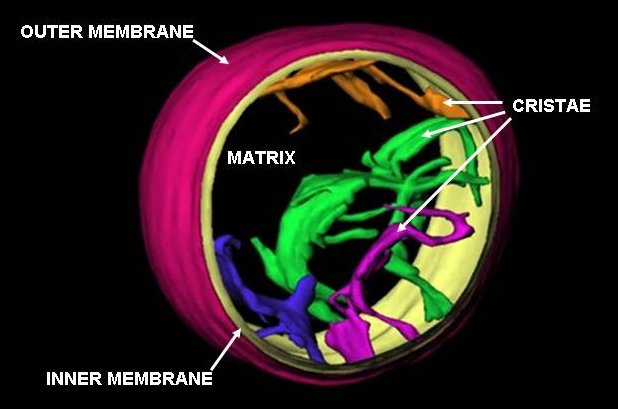
Mitochondrion
A mitochondrion () is an organelle found in the cell (biology), cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'', meaning a thread-like granule, was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 ''Scientific American'' article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). The multicellular animal ''Henneguya zschokkei, Henneguya salminicola'' is known to have retained mitochondrion-related organelles despite a complete loss of their mitochondrial genome. A large number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Horseradish Peroxidase
The enzyme horseradish peroxidase (HRP), found in the roots of horseradish, is used extensively in biochemistry applications. It is a metalloenzyme with many isoforms, of which the most studied type is C. It catalyzes the oxidation of various organic substrates by hydrogen peroxide. Structure The structure of the enzyme was first solved by X-ray crystallography in 1997; and has since been solved several times with various substrates. It is a large alpha-helix, alpha-helical glycoprotein which binds heme as a redox Cofactor (biochemistry), cofactor. Substrates Alone, the HRP enzyme, or conjugates thereof, is of little value; its presence must be made visible using a Substrate (biochemistry), substrate that, when Redox, oxidized by HRP using hydrogen peroxide as the oxidizing agent, yields a characteristic color change that is detectable by spectrophotometric methods. Numerous substrates for horseradish peroxidase have been described and commercialized to exploit the desir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ascorbate Peroxidase
Ascorbate peroxidase (or L-ascorbate peroxidase, APX or APEX) () is an enzyme that catalyzes the chemical reaction :L-ascorbate + H2O2 \rightleftharpoons dehydroascorbate + 2 H2O It is a member of the family of heme-containing peroxidases. Heme peroxidases catalyse the H2O2-dependent oxidation of a wide range of different, usually organic, substrates in biology. This enzyme belongs to the family of oxidoreductases, specifically those acting on a peroxide as acceptor (peroxidases). The systematic name of this enzyme class is L-ascorbate:hydrogen-peroxide oxidoreductase. Other names in common use include L-ascorbic acid peroxidase, L-ascorbic acid-specific peroxidase, ascorbate peroxidase, and ascorbic acid peroxidase. This enzyme participates in ascorbate and aldarate metabolism. Overview Ascorbate-dependent peroxidase activity was first reported in 1979,, more than 150 years after the first observation of peroxidase activity in horseradish plants and almost 40 years after the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphyrin
Porphyrins ( ) are heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (). In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis. The parent of porphyrins is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons the porphyrin ring structure is a coordinated aromatic system. One result of the large conjugated system is that porphyrins absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives . Structure Porphyrin complexes consist of a square planar MN4 core. The p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High Spin
Spin states when describing transition metal coordination complexes refers to the potential spin configurations of the central metal's d electrons. For several oxidation states, metals can adopt high-spin and low-spin configurations. The ambiguity only applies to first row metals, because second- and third-row metals are invariably low-spin. These configurations can be understood through the two major models used to describe coordination complexes; crystal field theory and ligand field theory (a more advanced version based on molecular orbital theory). High-spin vs. low-spin Octahedral complexes The Δ splitting of the ''d'' orbitals plays an important role in the electron spin state of a coordination complex. Three factors affect Δ: the period (row in periodic table) of the metal ion, the charge of the metal ion, and the field strength of the complex's ligands as described by the spectrochemical series. Only octahedral complexes of first row transition metals adopt hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptophan
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin). It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Tryptophan is named after the digestive enzymes trypsin, which were used in its first isolation from casein proteins. It was assigned the one-letter symbol W based on the double ring being visually suggestive to the bulky letter. Function ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Side Chain
In organic chemistry and biochemistry, a side chain is a substituent, chemical group that is attached to a core part of the molecule called the "main chain" or backbone chain, backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a larger hydrocarbon backbone. It is one factor in determining a molecule's properties and reactivity. A side chain is also known as a pendant chain, but a pendant group (side group) has a different definition. Conventions The placeholder R is often used as a generic placeholder for alkyl (saturated hydrocarbon) group side chains in structural formulae. To indicate other non-carbon groups in structure diagrams, X, Y, or Z are often used. History The ''R'' symbol was introduced by 19th-century French chemist Charles Frédéric Gerhardt, who advocated its adoption on the grounds that it would be widely recognizable and intelligible given its correspondence in multiple European languages to the initial letter of "r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free-radical
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mössbauer Spectroscopy
Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer (sometimes written "Moessbauer", German: "Mößbauer") in 1958, consists of the nearly recoil-free emission and absorption of nuclear gamma rays in solids. The consequent nuclear spectroscopy method is exquisitely sensitive to small changes in the chemical environment of certain nuclei. Typically, three types of nuclear interactions may be observed: the isomer shift due to differences in nearby electron densities (also called the chemical shift in older literature), quadrupole splitting due to atomic-scale electric field gradients; and magnetic splitting due to non-nuclear magnetic fields. Due to the high energy and extremely narrow line widths of nuclear gamma rays, Mössbauer spectroscopy is a highly sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting changes of just a few parts in 1011. It is a metho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferric
In chemistry, iron(III) or ''ferric'' refers to the chemical element, element iron in its +3 oxidation number, oxidation state. ''Ferric chloride'' is an alternative name for iron(III) chloride (). The adjective ''ferrous'' is used instead for iron(II) salts, containing the cation Fe2+. The word ''wikt:ferric, ferric'' is derived from the Latin word , meaning "iron". Although often abbreviated as Fe3+, that naked ion does not exist except under extreme conditions. Iron(III) centres are found in many compounds and coordination complexes, where Fe(III) is bonded to several Ligand, ligands. A molecular ferric complex is the anion ferrioxalate, , with three bidentate oxalate ions surrounding the Fe core. Relative to lower oxidation states, ferric is less common in organoiron chemistry, but the ferrocenium cation is well known. Iron(III) in biology All known forms of life require iron, which usually exists in Fe(II) or Fe(III) oxidation states. Many proteins in living beings cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |