|
Cycloaliphatic
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains (also known as monocycloalkanes) are classified as small (cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium (cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons that are polycyclic. In any case, the general form of the chemical formul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
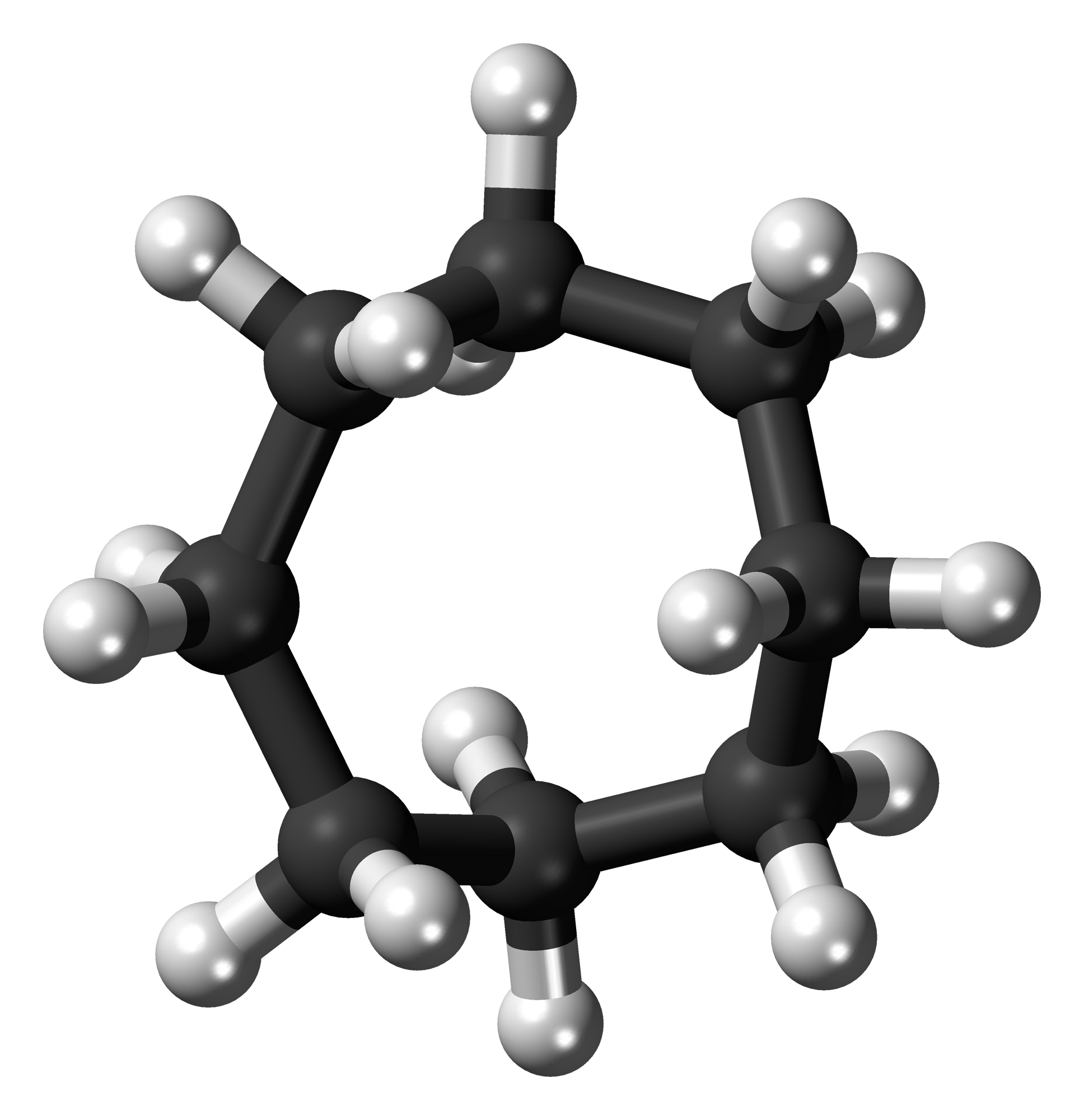
Cyclooctane
Cyclooctane is a cycloalkane with the molecular formula (CH2)8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general. Cyclooctane has a camphoraceous odor. Conformations The conformational isomer, conformation of cyclooctane has been studied extensively using computational chemistry, computational methods. Hendrickson noted that "cyclooctane is unquestionably the conformationally most complex cycloalkane owing to the existence of many conformers of comparable energy". The boat-chair conformation (below) is the most stable form. Allinger and co-workers confirmed this. The crown conformation (below) is slightly less stable. Among the many compounds exhibiting the crown conformation (structure II) is S8, elemental sulfur. : Synthesis and reactions The main route to cyclooctane derivatives involves the dimerization of butadiene, catalysed by nickel(0) complexes such as nickel bis(cyclooctadiene). This pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicyclic Compound
A bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings' atoms consist of at least two elements), like DABCO. Moreover, the two rings can both be aliphatic (''e.g.'' decalin and norbornane), or can be aromatic (''e.g.'' naphthalene), or a combination of aliphatic and aromatic (''e.g.'' tetralin). Three modes of ring junction are possible for a bicyclic compound: * In spiro compounds, the two rings share only one single atom, the spiro atom, which is usually a quaternary carbon. An example of a spirocyclic compound is the photochromic switch spiropyran. * In fused/condensed bicyclic compounds, two rings share two adjacent atoms. In other words, the rings share one covalent bond, ''i.e.'' the bridgehead atoms are directly connected (''e.g.'' α-thuje ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum refining, it is often a constituent of liquefied petroleum gas (LPG), which is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation; other constituents of LPG may include propene, propylene, butane, butene, butylene, butadiene, and isobutylene. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane has lower volumetric energy density than gasoline or coal, but has higher gravimetric energy density than them and burns more cleanly. Propane gas has become a popular choice for barbecues and portable stoves because its low −42 °C boiling point makes it vaporise inside pressurised liquid containers (it exists in two pha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like hexacontane () or 4-methyl-5-(1-methylethyl) octane, an isomer of dodecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula , and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote ''any'' saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes) or polycycl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctane
Cyclooctane is a cycloalkane with the molecular formula (CH2)8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general. Cyclooctane has a camphoraceous odor. Conformations The conformational isomer, conformation of cyclooctane has been studied extensively using computational chemistry, computational methods. Hendrickson noted that "cyclooctane is unquestionably the conformationally most complex cycloalkane owing to the existence of many conformers of comparable energy". The boat-chair conformation (below) is the most stable form. Allinger and co-workers confirmed this. The crown conformation (below) is slightly less stable. Among the many compounds exhibiting the crown conformation (structure II) is S8, elemental sulfur. : Synthesis and reactions The main route to cyclooctane derivatives involves the dimerization of butadiene, catalysed by nickel(0) complexes such as nickel bis(cyclooctadiene). This pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloheptane
Cycloheptane, also known as Suberane, is an Chemical compound, organic compound, which belongs to the group of cycloalkanes. The compound can occur in different Rotamer, conformers. Production Cycloheptane occurs naturally in petroleum and can be extracted from it. It is synthesized by a Clemmensen reduction from cycloheptanone. Properties Cycloheptane is a colorless liquid with a mild, aromatic odor. The boiling point at normal pressure is 119°C. The molar enthalpy of vaporization is 38.5 kJ mol−1. According to the Antoine equation, the vapor pressure function is given by log10(''P'') = ''A''−(''B''/(''T''+''C'')) (''P'' in bar, ''T'' in kelvins) with ''A'' = 3.97710, ''B'' = 1330.402 and ''C'' = −56.946 in the temperature range from 341.3 K to 432.2 K. In the solid phase, cycloheptane occurs in four Crystal polymorphism, polymorphic forms. The transformation temperatures for the conversion from form IV to form III are −138°C, from form III to form II −75°C and f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexane is mainly used for the industrial production of adipic acid and caprolactam, which are Precursor (chemistry), precursors to nylon. Cyclohexyl () is the alkyl substituent of cyclohexane and is abbreviated Cy. Production Cyclohexane is one of components of naphtha, from which it can be extracted by advanced distillation methods. Distillation is usually combined with isomerization of methylcyclopentane, a similar component extracted from naphtha by similar methods. Together, these processes cover only a minority (15-20%) of the modern industrial demand, and are complemented by synthesis. Modern industrial synthesis On an industrial scale, cyclohexane is produced by hydrogenation of benzene in the presence of a Raney nickel catalyst. Prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic compound, alicyclic hydrocarbon with chemical formula C5H10, C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It is a colorless liquid with a petrol-like odor. Its freezing point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more carbon rings. It is formed by cracking (chemistry), cracking cyclohexane in the presence of alumina at a high temperature and pressure. It was first prepared in 1893 by the German chemist Johannes Wislicenus. Production, occurrence and use Cycloalkanes are formed by catalytic reforming. For example, when passed over a hot platinum surface, 2-Methylbutane, 2-methylbutane converts into cyclopentane. Cyclopentane is principally used as a blowing agent in the manufacture of polyurethane foam, polyurethane insulating foam, replaci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology. Structure The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C. The four carbon atoms in cyclobutane are not coplanar; instead, the ring typically adopts a folded or "puckered" conformation. This implies that the C-C-C angle is less than 90°. One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way, some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives - cyclopropanes - are of commercial or biological significance. Cyclopropane was used as a clinical inhalational anesthetic from the 1930s through the 1980s. The substance's high flammability poses a risk of fire and explosions in operating rooms due to its tendency to accumulate in confined spaces, as its density is higher than that of air. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-Dibromopropane, 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |