|
Cyanines
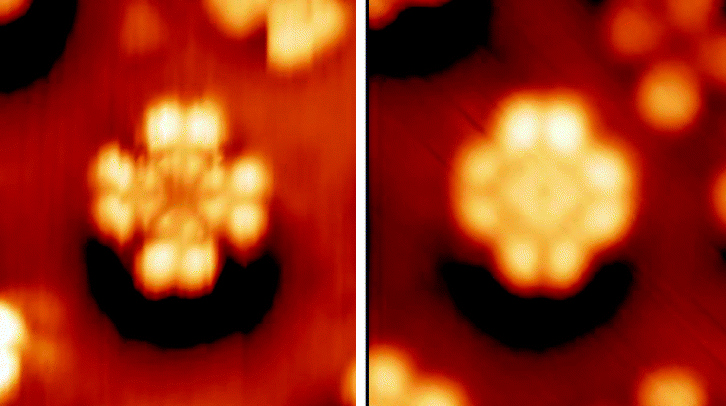
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV. Chemically, cyanines are a conjugated system between two nitrogen atoms; in each resonance structure, exactly one nitrogen atom is oxidized to an iminium. Typically, they form part of a nitrogenous heterocyclic system. The main application for cyanine dyes is in biological labeling. Nevertheless, there is a wide literature on both their synthesis and uses, and cyanines are common in some CD and DVD media. Structure Cyanines have been classified in many ways: * ''Streptocyanines'' or ''open chain cyanines'': : R2N+=CH H=CH'n''-NR2 (I) * ''Hemicyanines'': : Aryl=N+=CH H=CH'n''-NR2 (II) * ''Closed chain cyanines'': :Aryl=N+=CH H=CH'n''-N=Aryl (III) Additionally, these classes are recognized: * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phthalocyanine
Phthalocyanine () is a large, aromatic, macrocyclic, organic compound with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity. It is composed of four isoindole units linked by a ring of nitrogen atoms. = has a two-dimensional geometry and a ring system consisting of 18 π-electrons. The extensive delocalization of the π-electrons affords the molecule useful properties, lending itself to applications in dyes and pigments. Metal complexes derived from , the conjugate base of , are valuable in catalysis, organic solar cells, and photodynamic therapy. Properties Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents. Benzene at 40 °C dissolves less than a milligram of or CuPc per litre. and CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are, thermally, very stable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanine
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV. Chemically, cyanines are a conjugated system between two nitrogen atoms; in each resonance structure, exactly one nitrogen atom is oxidized to an iminium. Typically, they form part of a nitrogenous heterocyclic system. The main application for cyanine dyes is in biological labeling. Nevertheless, there is a wide literature on both their synthesis and uses, and cyanines are common in some CD and DVD media. Structure Cyanines have been classified in many ways: * ''Streptocyanines'' or ''open chain cyanines'': : R2N+=CH H=CH'n''-NR2 (I) * ''Hemicyanines'': : Aryl=N+=CH H=CH'n''-NR2 (II) * ''Closed chain cyanines'': :Aryl=N+=CH H=CH'n''-N=Aryl (III) Additionally, these classes are recognized: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiropyran
A spiropyran is a type of organic chemical compound, known for photochromic properties that provide this molecule with the ability of being used in medical and technological areas. Spiropyrans were discovered in the early twentieth century. However, it was in the middle twenties when Fisher and Hirshbergin observed their photochromic characteristics and reversible reaction. In 1952, Fisher and co-workers announced for the first time photochromism in spiropyrans. Since then, there have been many studies on photochromic compounds that have continued up to the present. Synthesis There are two methods for the production of spiropyrans. The first one can be by condensation of methylene bases with o-hydroxy aromatic aldehydes (or the condensation of the precursor of methylene bases). Spiropyrans generally could be obtained by boiling the aldehyde and the respective benzazolium salts in presence of pyridine or piperidine. The general formula of the synthesis of spiropyrans is shown in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymethine
Polymethines are compounds made up from an ''odd'' number of methine groups (CH) bound together by alternating single and double bonds.Kachovski and Dekhtyar, ''Dyes and Pigments'', 22 (1983) 83-97. Compounds made up from an ''even'' number of methine groups are known as polyenes. Polymethine dyes Cyanines are synthetic dyes belonging to polymethine group. Anthocyanidins are natural plant pigments belonging to the group of the polymethine dyes. Polymethines are fluorescent dyes that may be attached to nucleic acid probes for different uses, ''e.g.'', to accurately count reticulocyte Reticulocytes are immature red blood cells (RBCs). In the process of erythropoiesis (red blood cell formation), reticulocytes develop and mature in the bone marrow and then circulate for about a day in the blood stream before developing into mat ...s. References Alkenes {{alkene-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group. Oxazoles are related compounds, with sulfur replaced by oxygen. Thiazoles are structurally similar to imidazoles, with the thiazole sulfur replaced by nitrogen. Thiazole rings are planar and aromatic. Thiazoles are characterized by larger pi-electron delocalization than the corresponding oxazoles and have therefore greater aromaticity. This aromaticity is evidenced by the chemical shift of the ring protons in proton NMR spectroscopy (between 7.27 and 8.77 ppm), clearly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleic Acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is the ribose derivative deoxyribose, the polymer is DNA. Nucleic acids are naturally occurring chemical compounds that serve as the primary information-carrying molecules in cells and make up the genetic material. Nucleic acids are found in abundance in all living things, where they create, encode, and then store information of every living cell of every life-form on Earth. In turn, they function to transmit and express that information inside and outside the cell nucleus to the interior operations of the cell and ultimately to the next generation of each living organism. The encoded informatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Compound
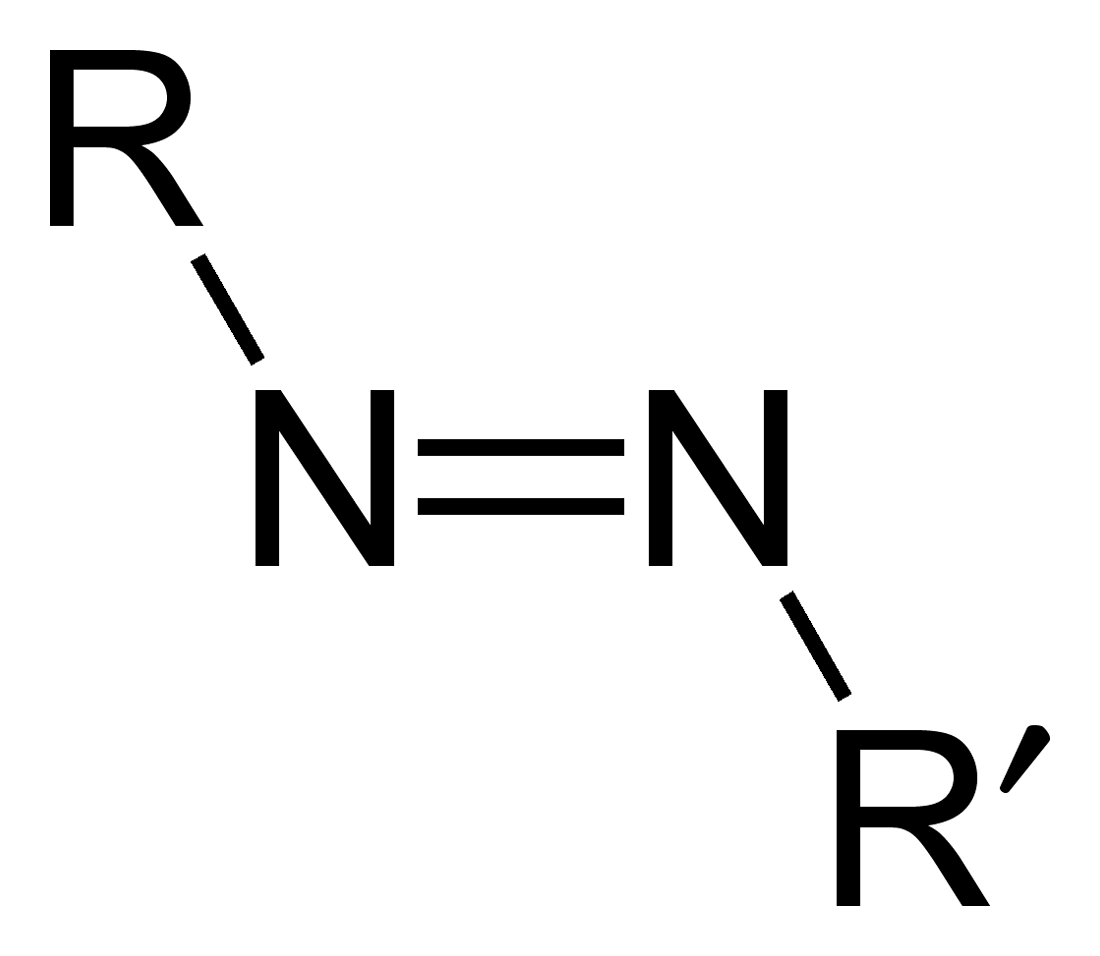
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diaz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DVD-R
DVD recordable and DVD rewritable are optical disc recording technologies. Both terms describe DVD optical discs that can be written to by a DVD recorder, whereas only 'rewritable' discs are able to erase and rewrite data. Data is written ('burned') to the disc by a laser, rather than the data being 'pressed' onto the disc during manufacture, like a DVD-ROM. Pressing is used in mass production, primarily for the distribution of home video. Like CD-Rs, DVD recordable uses dye to store the data. During the burning of a single bit, the laser's intensity affects the reflective properties of the burned dye. By varying the laser intensity quickly, high density data is written in precise tracks. Since written tracks are made of darkened dye, the data side of a recordable DVD has a distinct color. Burned DVDs have a higher failure-to-read rate than pressed DVDs, due to differences in the reflective properties of dye compared to the aluminum substrate of pressed discs. Comparing r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD-R
CD-R (Compact disc-recordable) is a digital optical disc storage format. A CD-R disc is a compact disc that can be written once and read arbitrarily many times. CD-R discs (CD-Rs) are readable by most CD readers manufactured prior to the introduction of CD-R, unlike CD-RW discs. History Originally named CD Write-Once (WO), the CD-R specification was first published in 1988 by Philips and Sony in the Orange Book, which consists of several parts that provide details of the CD-WO, CD-MO (Magneto-Optic), and later CD-RW (ReWritable). The latest editions have abandoned the use of the term "CD-WO" in favor of "CD-R", while " CD-MO" was rarely used. Written CD-Rs and CD-RWs are, in the aspect of low-level encoding and data format, fully compatible with the audio CD (''Red Book'' CD-DA) and data CD (''Yellow Book'' CD-ROM) standards. The Yellow Book standard for CD-ROM only specifies a high-level data format and refers to the Red Book for all physical format and low-level code ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Panchromatic
Panchromatic emulsion is a type of black-and-white photographic emulsion that is sensitive to all wavelengths of visible light. Description A panchromatic emulsion renders a realistic reproduction of a scene as it appears to the human eye, although with no colors. Almost all modern photographic film is panchromatic. Some older types of film were orthochromatic and were not sensitive to certain wavelengths of light. As naturally prepared, a silver halide photographic emulsion is much more sensitive to blue and UV light than to green and red wavelengths. The German chemist Hermann W. Vogel found out how to extend the sensitivity into the green, and later the orange, by adding sensitising dyes to the emulsion. By the addition of erythrosine the emulsion could be made orthochromatic while some cyanine derivatives confer sensitivity to the whole visible spectrum making it panchromatic. However, his technique was not extended to achieve a fully panchromatic film until the early 1900 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats. It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, troughs, or zero crossings, and is a characteristic of both traveling waves and standing waves, as well as other spatial wave patterns. The inverse of the wavelength is called the spatial frequency. Wavelength is commonly designated by the Greek letter '' lambda'' (λ). The term ''wavelength'' is also sometimes applied to modulated waves, and to the sinusoidal envelopes of modulated waves or waves formed by interference of several sinusoids. Assuming a sinusoidal wave moving at a fixed wave speed, wavelength is inversely proportional to frequency of the wave: waves with higher frequencies have shorter wavelengths, and lower frequencies have longer wavelengths. Wavelength depends on the medium (for example, vacuum, air, or water) that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photo Emulsion
Photographic emulsion is a light-sensitive colloid used in film-based photography. Most commonly, in silver-gelatin photography, it consists of silver halide crystals dispersed in gelatin. The emulsion is usually coated onto a substrate of glass, films (of cellulose nitrate, cellulose acetate or polyester), paper, or fabric. Photographic emulsion is not a true emulsion, but a suspension of solid particles (silver halide) in a fluid (gelatin in solution). However, the word ''emulsion'' is customarily used in a photographic context. Gelatin or gum arabic layers sensitized with dichromate used in the dichromated colloid processes carbon and gum bichromate are sometimes called ''emulsions''. Some processes do not have emulsions, such as platinum, cyanotype, salted paper, or kallitype. Components Photographic emulsion is a fine suspension of insoluble light-sensitive crystals in a colloid sol, usually consisting of gelatin. The light-sensitive component is one or a mixture of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |