|
Cork Taint
Cork taint is a broad term referring to an off-odor and off-flavor wine fault arising from the presence of 2,4,6-trichloroanisole (TCA), a chemical compound that represents one of the strongest off-flavors, and one "generated naturally in foods/beverages", in particular wines, that considerably reduces the quality of these products. Cork taint is characterized by a set of undesirable smells or tastes found in a bottle of wine, especially spoilage that can only be detected after bottling, aging and opening. Though modern studies have shown that other factors can also be responsible for taint—including wooden barrels, storage conditions and the transport of corks and wine—the cork stopper is normally considered to be responsible, and a wine found to be tainted on opening is said to be corked or "corky". Cork taint can affect wines irrespective of price and quality level. The chief cause of cork taint is the presence of the chemical compounds 2,4,6-trichloroanisole (TCA) or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Habituation
Habituation is a form of non-associative learning in which an organism’s non-reinforced response to an inconsequential stimulus decreases after repeated or prolonged presentations of that stimulus. For example, organisms may habituate to repeated sudden loud noises when they learn that these have no consequences. Habituation can occur in responses that habituate include those that involve an entire organism or specific biological component systems of an organism. The broad ubiquity of habituation across all forms of life has led to it being called "the simplest, most universal form of learning...as fundamental a characteristic of life as DNA." Functionally, habituation is thought to free up cognitive resources for other stimuli that are associated with biologically important events by diminishing the response to inconsequential stimuli. A progressive decline of a behavior in a habituation procedure may also reflect nonspecific effects such as fatigue, which must be ruled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
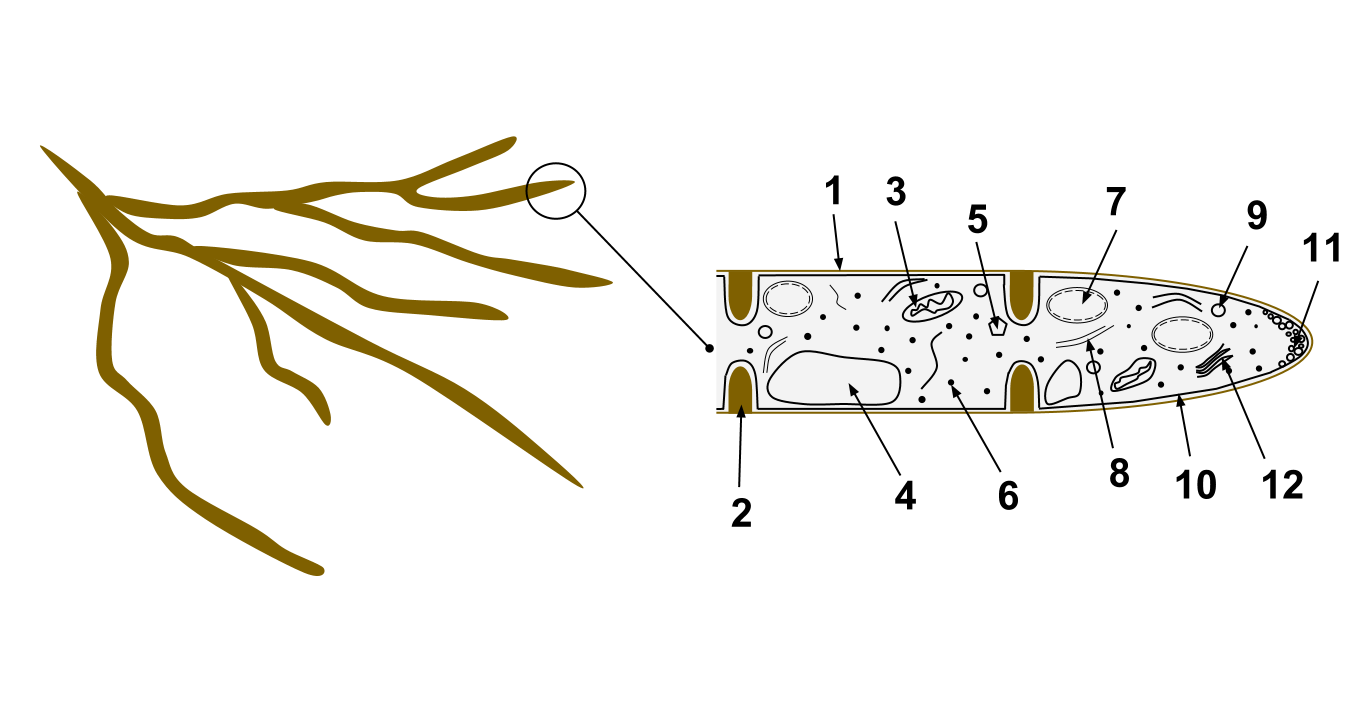
Fungus
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one of the kingdom (biology)#Six kingdoms (1998), traditional eukaryotic kingdoms, along with Animalia, Plantae, and either Protista or Protozoa and Chromista. A characteristic that places fungi in a different kingdom from plants, bacteria, and some protists is chitin in their cell walls. Fungi, like animals, are heterotrophs; they acquire their food by absorbing dissolved molecules, typically by secreting digestive enzymes into their environment. Fungi do not photosynthesize. Growth is their means of motility, mobility, except for spores (a few of which are flagellated), which may travel through the air or water. Fungi are the principal decomposers in ecological systems. These and other differences place fungi in a single group of related o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophenol
A chlorophenol is any organochloride of phenol that contains one or more covalent, covalently bonded chlorine atoms. There are five basic types of chlorophenols (mono- to pentachlorophenol) and 19 different chlorophenols in total when positional isomerism is taken into account. Chlorophenols are produced by electrophilic halogenation of phenol with chlorine. Most chlorophenols are solid at room temperature. They have a strong, medicinal taste and smell. Chlorophenols are commonly used as pesticides, herbicides, and disinfectants. List of chlorophenols There is a total of 19 chlorophenols, corresponding to the different ways in which chlorine atoms can be attached to the five carbons in the benzene ring of the phenol molecule, excluding the carbon atom to which the hydroxy group is attached. Monochlorophenols have three isomers because there is only one chlorine atom that can occupy one of three ring positions on the phenol molecule; 2-chlorophenol, for example, is the isomer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine Dioxide
Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually handled as an aqueous solution. It is commonly used as a bleach. More recent developments have extended its applications in food processing and as a disinfectant. Structure and bonding The molecule ClO2 has an odd number of valence electrons, and therefore it is a Paramagnetism, paramagnetic radical (chemistry), radical. It is an unusual "example of an odd-electron molecule stable toward dimerization" (nitric oxide being another example). ClO2 crystallizes in the orthorhombic List of space groups, Pbca space group. History Chlorine dioxide was first prepared in 1811 by Sir Humphry Davy. In 1933, Lawrence O. Brockway, a graduate student of Linus Pauling, proposed a structure that involved a three-electron bond and two single bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peracetic Acid
Peracetic acid (also known as peroxyacetic acid, or Percidine) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive. Peracetic acid is a weaker acid than the parent acetic acid, with a p''K''a of 8.2. Production Peracetic acid is produced industrially by the autoxidation of acetaldehyde: :O2 + CH3CHO → CH3CO3H Peracetic acid is conventionally prepared by combining acetic acid and hydrogen peroxide with homogeneous acid catalysts (e.g., sulfuric acid), which facilitate the reaction and achieve equilibrium between the reagents and product: :H2O2 + CH3CO2H CH3CO3H + H2O While it is feasible to create peracetic acid by combining consumer-grade vinegar (5% acetic acid) and hydrogen peroxide (3%) without an acid catalyst, the low concentration of reagents will result in a slow reaction rate at room temperature. Extrapolating from published reaction rates, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined to each other and to adjacent elements through Single bond, single covalent bonds, denoted by dashes or lines. The group in a peroxide is often called the peroxide group, though some nomenclature discrepancies exist. This linkage is recognized as a common polyatomic ion, and exists in many molecules. General structure The characteristic structure of any regular peroxide is the oxygen–oxygen covalent single bond, which connects the two main atoms together. In the event that the molecule has no chemical Substituent, substituents, the peroxide group will have a [−2] Formal charge, net charge. Each oxygen atom has a charge of negative one, as 5 of its Valence electron, valence electrons remain in the outermost Atomic orbital, orbital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pallet
A pallet (also called a skid) is a flat transport structure, which supports goods in a stable fashion while being lifted by a forklift, a pallet jack, a Loader (equipment), front loader, a Jack (mechanical), jacking device, or an erect crane. Many pallets can handle a load of . While most pallets are wooden, pallets can also be made of plastic, metal, paper, and recycled materials. A pallet is the structural foundation of a unit load, which allows handling and storage efficiencies. Goods in shipping containers are often placed on a pallet secured with strapping, stretch wrap or shrink wrap and shipped. In addition, pallet collars can be used to support and protect items shipped and stored on pallets. Containerization for transport has spurred the use of pallets because shipping containers have the smooth, level surfaces needed for easy pallet movement. Since its invention in the twentieth century, its use has dramatically supplanted older forms of crating like the wooden b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barrel
A barrel or cask is a hollow cylindrical container with a bulging center, longer than it is wide. They are traditionally made of wooden stave (wood), staves and bound by wooden or metal hoops. The word vat is often used for large containers for liquids, usually alcoholic beverages; a small barrel or cask is known as a keg. Barrels have a variety of uses, including storage of liquids such as water, oil, and alcohol. They are also employed to hold maturing beverages such as wine, Cognac (brandy), cognac, Armagnac (drink), armagnac, sherry, port wine, port, whiskey, beer, arrack, and sake. Other commodities once stored in wooden casks include gunpowder, Salt-cured meat, meat, fish, paint, honey, nails, and tallow. Modern wooden barrels for wine-making are made of English oak (''Quercus robur''), white Oak (wine), oak (''Quercus petraea''), American white oak (''Quercus alba''), more exotic is mizunara oak (''Quercus crispula''), and recently Oregon oak (''Quercus garryana'') ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypochlorous Acid
Hypochlorous acid is an inorganic compound with the chemical formula , also written as HClO, HOCl, or ClHO. Its structure is . It is an acid that forms when chlorine dissolves in water, and itself partially dissociates, forming a hypochlorite anion, . HClO and are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor, chlorine. Because of its strong antimicrobial properties, the related compounds sodium hypochlorite (NaOCl) and calcium hypochlorite () are ingredients in many commercial bleaches, deodorants, and disinfectants. The white blood cells of mammals, such as humans, also contain hypochlorous acid as a tool against foreign bodies. In living organisms, HOCl is generated by the reaction of hydrogen peroxide with chloride ions under the catalysis of the heme enzyme myeloperoxidase (MPO). Like many other disinfectants, hypochlorous acid solutions will destroy pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |