|
Chromium(VI)
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is chromium in any chemical compound that contains the element in the +6 oxidation state (thus hexavalent). Virtually all chromium ore is processed via hexavalent chromium, specifically the salt sodium dichromate. Hexavalent chromium is key to all materials made from chromium. Approximately of hexavalent chromium were produced in 1985. Additional hexavalent chromium compounds include chromium trioxide and various salts of chromate and dichromate, among others. Hexavalent chromium is used in textile dyes, wood preservation, anti-corrosion products, chromate conversion coatings, and a variety of niche uses. Industrial uses of hexavalent chromium compounds include chromate pigments in dyes, paints, inks, and plastics; chromates added as anticorrosive agents to paints, primers, and other surface coatings; and chromic acid electroplated onto metal parts to provide a decorative or protective coating. Hexavalent chromium can be fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal. Chromium metal is valued for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Chromium is also greatly valued as a metal that is able to be highly polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared light. The name of the element is derived from the Greek word χρῶμα, ''chrōma'', meaning color, because many chromium compounds are intensely colored. Industrial production of chromium proceeds from chromite ore (mostly FeCr2O4) to pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium Trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser and a carcinogen. Production, structure, and basic reactions Chromium trioxide is generated by treating sodium dichromate with sulfuric acid: :H2SO4 + Na2Cr2O7 → 2 CrO3 + Na2SO4 + H2O Approximately 100,000 tonnes are produced annually by this or similar routes. The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3. : Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromate And Dichromate
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible. Chemical properties Potassium-chromate-sample.jpg, potassium chromate Potassium-dichromate-sample.jpg, potassium dichromate Chromates react with hydrogen peroxide, giving products in which peroxide, , replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, CrO(O2)2, is formed; it is an uncharged covalent molecule, which may be extracted into ether. Addition of pyridine results in the formation of the more stable complex CrO(O2)2py. Acid–base properties In aqueous solution, chromate and dichromate anions exist in a chemical equilibrium. :2 + 2 H+ + H2O The predominance diagram shows that the position of the equilibrium depends ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IARC Group 1
Substances, mixtures, and exposure circumstances in this list have been classified as group 1 by the International Agency for Research on Cancer (IARC): The agent (mixture) is carcinogenic to humans. The exposure circumstance entails exposures that are carcinogenic to humans. This category is used when there is sufficient evidence of carcinogenicity in humans. Exceptionally, an agent (mixture) may be placed in this category when evidence of carcinogenicity in humans is less than sufficient but there is sufficient evidence of carcinogenicity in experimental animals and strong evidence in exposed humans that the agent (mixture) acts through a relevant mechanism of carcinogenicity. Agents Infectious conditions Viruses *Human immunodeficiency virus type 1 (infection with) * Human T-cell lymphotropic virus type I *Human papillomavirus types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 *Hepatitis B virus (chronic infection with) *Hepatitis C virus (chronic infection with) * Ka ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious. Carcinogens, as mentioned, are agents in the environment capable of contributing to cancer growth. Carcinogens can be categorized into two different types: activation-dependent and activation-independent, and each nature impacts their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genotoxicity
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic substances are not mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis. To assay for genotoxic molecules, researchers assay for DNA damage in cells exposed to the toxic substrates. This DNA damage can be in the form of single- and double-strand breaks, loss of excision repair, cross-linking, alkali-labile sites, point mutations, and st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
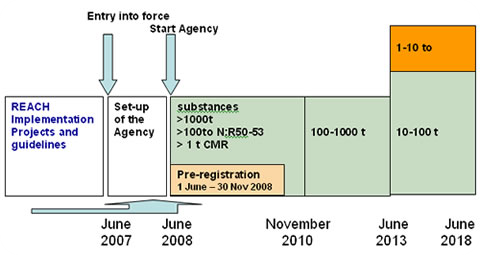
Registration, Evaluation, Authorisation And Restriction Of Chemicals
Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH. Overview When REACH is fully in force, it will require all companies manufacturing or importing chemical substances into the European Union in quantities of one tonne or more per year to register these substances with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Restriction Of Hazardous Substances Directive
The Restriction of Hazardous Substances Directive 2002/95/EC (RoHS 1), short for Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment, was adopted in February 2003 by the European Union. The initiative was to prevent an overabundance of chemicals in electronics. Thus, as a result electronics were restricted. The RoHS 1 directive took effect on 1 July 2006, and is required to be enforced and became a law in each member state. This directive restricts (with exceptions) the use of ten hazardous materials in the manufacture of various types of electronic and electrical equipment. In addition to the exceptions, there are exclusions for products such as solar panels. It is closely linked with the Waste Electrical and Electronic Equipment Directive (WEEE) 2002/96/EC (now superseded) which sets collection, recycling and recovery targets for electrical goods and is part of a legislative initiative to solve the problem of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic ion, polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salt (chemistry), salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by International Union of Pure and Applied Chemistry, IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedron, tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge (physics), charge of −2 and it is the conjugate acid, conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Union
The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been described as a ''sui generis'' political entity (without precedent or comparison) combining the characteristics of both a federation and a confederation. Containing 5.8per cent of the world population in 2020, the EU generated a nominal gross domestic product (GDP) of around trillion in 2021, constituting approximately 18per cent of global nominal GDP. Additionally, all EU states but Bulgaria have a very high Human Development Index according to the United Nations Development Programme. Its cornerstone, the Customs Union, paved the way to establishing an internal single market based on standardised legal framework and legislation that applies in all member states in those matters, and only those matters, where the states have agree ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olfactory Epithelium
The olfactory epithelium is a specialized epithelial tissue inside the nasal cavity that is involved in smell. In humans, it measures 9 cm2 and lies on the roof of the nasal cavity about 7 cm above and behind the nostrils. The olfactory epithelium is the part of the olfactory system directly responsible for detecting odors. Structure Olfactory epithelium consists of four distinct cell types: * Olfactory sensory neurons * Supporting cells * Basal cells * Brush cells Olfactory sensory neurons The olfactory receptor neurons are sensory neurons of the olfactory epithelium. They are bipolar neurons and their apical poles express odorant receptors on non-motile cilia at the ends of the dendritic knob, which extend out into the airspace to interact with odorants. Odorant receptors bind odorants in the airspace, which are made soluble by the serous secretions from olfactory glands located in the lamina propria of the mucosa.Ross, MH, ''Histology: A Text and Atlas'', 5th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Agency For Research On Cancer
The International Agency for Research on Cancer (IARC; french: Centre International de Recherche sur le Cancer, CIRC) is an intergovernmental agency forming part of the World Health Organization of the United Nations. Its role is to conduct and coordinate research into the causes of cancer. It also collects and publishes surveillance data regarding the occurrence of cancer worldwide. Its IARC monographs programme identifies carcinogenic hazards and evaluates environmental causes of cancer in humans. IARC has its own governing council, and in 1965 the first members were the Federal Republic of Germany, France, Italy, the United Kingdom, and the United States of America. Today, IARC's membership has grown to 27 countries. History In late February 1963, after he experienced his spouse suffering and dying of cancer, journalist and peace activist Yves Poggioli sent a letter to Emmanuel d'Astier de la Vignerie relating his story, and urging support for the creation of an inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-oxid.jpg)