|
Chromates
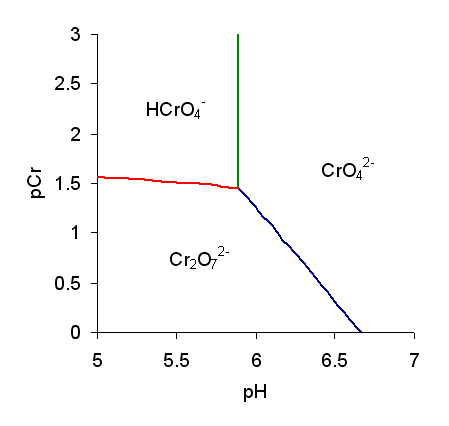
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible. Chemical properties Potassium-chromate-sample.jpg, Potassium chromate Potassium-dichromate-sample.jpg, Potassium dichromate Chromates react with hydrogen peroxide, giving products in which peroxide, , replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, , is formed; it is an uncharged covalent molecule, which may be extracted into ether. Addition of pyridine results in the formation of the more stable complex . Acid–base properties In aqueous solution, chromate and dichromate anions exist in a chemical equilibrium. : The predominance diagram shows that the position of the equilibrium depends on both pH and the analytical concentration o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal. Chromium is valued for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Chromium is also greatly valued as a metal that is able to be highly polishing, polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared, infrared light. The name of the element is derived from the Ancient Greek, Greek word χρῶμα, ''chrōma'', meaning color, because many chromium compounds are intensely colored. Indust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexavalent Chromium
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is any chemical compound that contains the element chromium in the +6 oxidation state (thus hexavalent). It has been identified as carcinogenic, which is of concern since approximately of hexavalent chromium were produced in 1985. Hexavalent chromium compounds can be carcinogens ( IARC Group 1), especially if airborne and inhaled where they can cause lung cancer. Occurrence and uses Hexavalent chromium occurs only rarely in nature, an exception being crocoite (PbCrO4). It is however produced on a large scale industrially. Virtually all chromium ore is processed via the formation of hexavalent chromium, specifically the salt sodium dichromate. Sodium chromate is converted into other hexavalent chromium compounds such as chromium trioxide and various salts of chromate and dichromate. Industrial uses of hexavalent chromium compounds include chromate pigments in dyes, paints, inks, and plastics; chromates added as anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromic Acid
Chromic acid is a chemical compound with the chemical formula . It is also a jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide. The term "chromic acid" is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the molecular species, of which the trioxide is the anhydride. Chromic acid features chromium in an oxidation state of +6 (and a valence of VI or 6). It is a strong and corrosive oxidizing agent and a moderate carcinogen. Molecular chromic acid Molecular chromic acid, , in principle, resembles sulfuric acid, . It would ionize accordingly: : The p''K''a for the equilibrium is not well characterized. Reported values vary between about −0.8 to 1.6. The structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Chromate
Potassium chromate is the inorganic compound with the formula Potassium, K2Chromate ion, CrO4. This yellow solid is the potassium salt of the Chromate ion, chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially. Structure Two crystalline forms are known, both being very similar to the corresponding potassium sulfate. Orthorhombic β-K2CrO4 is the common form, but it converts to an α-form above 666 °C.Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinger, "Chromium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. These structures are complex, although the chromate ion adopts the typical tetrahedral geometry.Gaultier, M.; Pannetier, G. "Structure cristalline de la forme 'basse temperature' du sulfate de potassium K2SO4-beta" (Crystal structure of the "low temperature" β-form of potassium sulfate) Bulletin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(VI) Oxide Peroxide
Chromium(VI) oxide peroxide is a chemical compound with the chemical formula . The name "chromium(VI) oxide peroxide" is also given to a collection of chromium coordination complexes. They have the formula where L is a ligand. These species are dark blue and often labile. They all feature oxo ligand and two peroxo ligands, with the remaining coordination sites occupied by water, hydroxide, diethyl ether, pyridine, or other Lewis bases. Preparation and properties Chromium(VI) oxide peroxide is formed by the addition of acidified hydrogen peroxide solutions to solutions of metal chromates or dichromates, such as sodium chromate or potassium dichromate. The generally yellow chromates or orange dichromates turn to dark blue as "chromium(VI) oxide peroxide" forms: : The structure of the pyridine complex has been determined crystallographically. Adducts with other N-heterocycles have also been characterized similarly. Aqueous chromium(VI) oxide peroxide decomposes in a few seco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Dichromate
Potassium dichromate is the inorganic compound with the formula . An orange solid, it is used in diverse laboratory and industrial applications. As with all hexavalent chromium compounds, it is chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinger, "Chromium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Production Potassium dichromate is usually prepared by the reaction of sodium dichromate and potassium chloride. Alternatively, it can be also obtained from potassium chromate by roasting chromite ore with potassium hydroxide: : Structure The solid crystallizes as two polymorphs. These salts are soluble in water, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(III) Hydroxide
Chromium(III) hydroxide is a gelatinous green inorganic compound with the chemical formula . It is a polymer with an undefined structure and low solubility. It is amphoteric, dissolving in both strong alkalis and strong acids. :In alkali: :In acid: It is used as a pigment, as a mordant, and as a catalyst for organic reactions. It is manufactured by adding a solution of ammonium hydroxide Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although ... to a solution of chromium salt. Pure is as yet (2020) unknown among the mineral species. However, three natural polymorphs of the chromium(III) oxide hydroxide, CrO(OH), are known: bracewellite, grimaldiite and guyanaite. References Chromium(III) compounds Hydroxides Chromium–oxygen compounds {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox Potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is expressed in volts (V). Each species has its own intrinsic redox potential; for example, the more positive the reduction potential (reduction potential is more often used due to general formalism in electrochemistry), the greater the species' affinity for electrons and tendency to be reduced. Measurement and interpretation In aqueous solutions, redox potential is a measure of the tendency of the solution to either gain or lose electrons in a reaction. A solution with a higher (more positive) reduction potential than some other molecule will have a tendency to gain electrons from this molecule (i.e. to be reduced by oxidizing this other molecule) and a solution with a lower (more negative) reduction potential w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Predominance Diagram Cr
''Predominance'' is the debut album by the Norwegian thrash metal band Susperia. Produced at the Abyss Studios, it combines thrash metal with black metal. Track listing #"I Am Pain" – 4:34 #"Vainglory" – 4:35 #"Illusions of Evil" – 5:44 #"Specimen" – 3:57 #"Journey into Black" – 3:50 #"Of Hate We Breed" – 4:57 #"Objects of Desire" – 4:03 #"The Hellchild" – 4:43 #"Blood on My Hands" – 5:14 #"The Coming of a Darker Time" – 3:34 Personnel * Athera – vocals *Cyrus – lead guitar *Elvorn – rhythm guitar * Memnock – bass *Tjodalv Ian Kenneth Åkesson (born 1976), known professionally as Tjodalv, is a Norwegian drummer. He is a founding member of the black metal bands Dimmu Borgir and Old Man's Child. He now plays in Black Comedy and Susperia. Tjodalv played guitar on th ... – drums References {{Authority control 2001 debut albums Susperia albums ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous Solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. Characteristics Substances that are ''hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are '' hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chloride. In an aqueous solution the hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reduction (chemistry)
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |