|
Cholinesterase Reactivator
Cholinesterase reactivators are drugs that reverse the inhibition of cholinesterase by organophosphates or sulfonates. They are used as antidote for treating organophosphate insecticide and nerve agent poisoning. Organophosphates are used industrially in agricultural pesticides, and globally as agents of chemical warfare. Discovered in 1955, Pralidoxime was the first cholinesterase reactivator used as an antidote to OP neurotoxicity and remains the most commonly used reactivator. Cholinesterase reactivators are indicated for anticholinesterase toxicity and cholinergic crisis, the signs of which are contained within the mnemonic ''DUMBELS'' : Diarrhea/diaphoresis, urinary frequency, miosis, bronchospasm/bronchorrhea, emesis, lacrimation, and salivation. Death may occur due to the blockage of nicotinic receptors of muscle fibers at the neuromuscular junction, causing paralysis of the muscles of respiration. Mechanism of action Organophosphates lead to toxicity by forming a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholinesterase
The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters: : an acylcholine + H2O = choline + a carboxylate Several of these serve as neurotransmitters. Thus, it is either of two enzymes that catalyze the hydrolysis of these cholinergic neurotransmitters, such as breaking acetylcholine into choline and acetic acid. These reactions are necessary to allow a cholinergic neuron to return to its resting state after activation. For example, in muscle contraction, acetylcholine at a neuromuscular junction triggers a contraction; but for the muscle to relax afterward, rather than remaining locked in a tense state, the acetylcholine must be broken down by a choline esterase. The main type for that purpose is acetylcholinesterase (also called choline esterase I or erythrocyte cholinesterase); it is found mainly in chemical synapses and red blood cell membranes. The other type is butyrylcholin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
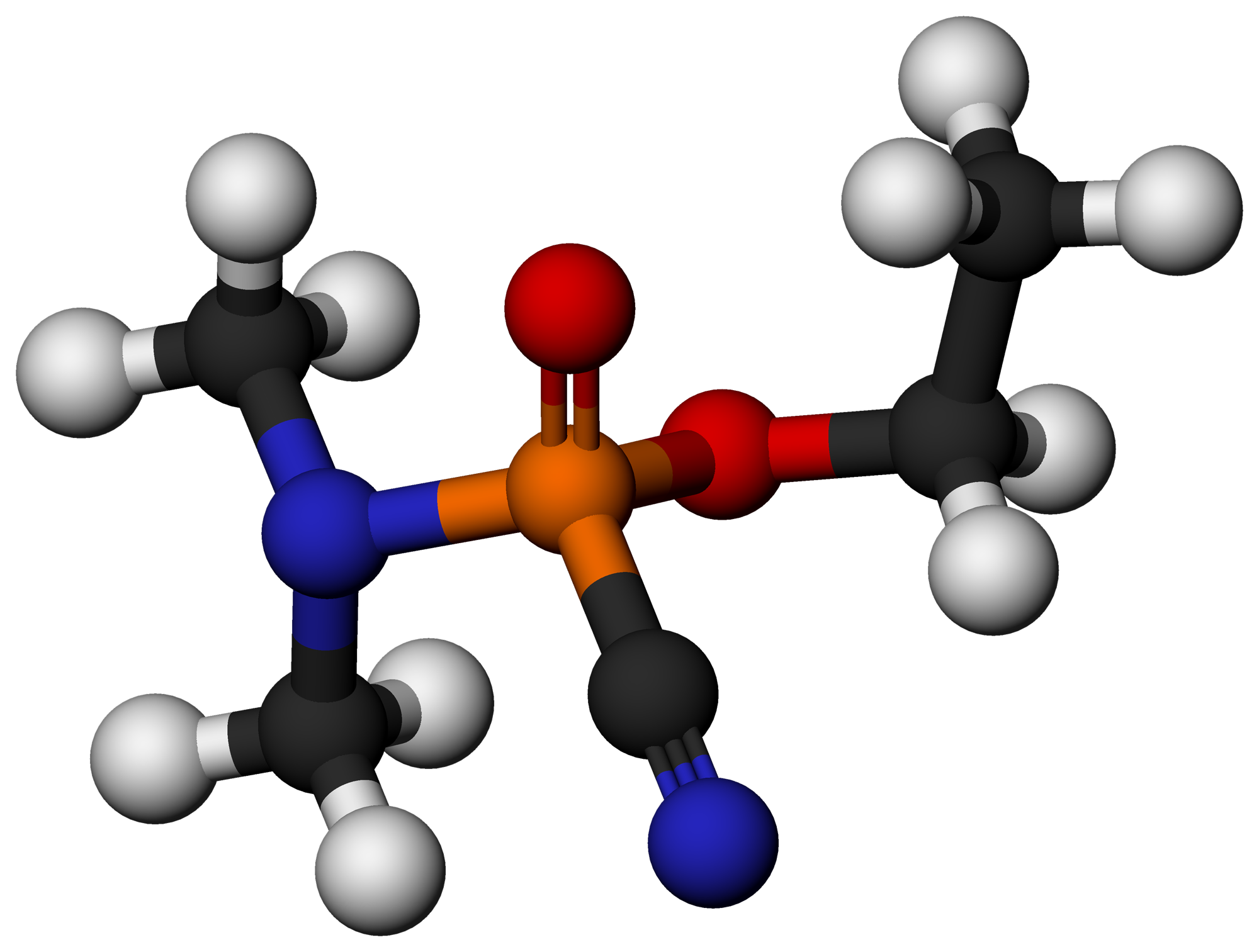
Organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Organophosphates are best known for their use as pesticides. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product physically rather than by chemical bond. Due to this, OPEs leak in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonate
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non- oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates. Sulfonate salts Anions with the general formula are called sulfonates. They are the conjugate bases of sulfonic acids with formula . As sulfonic acids tend to be strong acids, the corresponding sulfonates are weak bases. Due to the stability of sulfonate anions, the cations of sulfonate salts such as scandium triflate have application as Lewis acids. A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst: : An alternative is the condensation of a sulfonyl ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antidote
An antidote is a substance that can counteract a form of poisoning. The term ultimately derives from the Greek term φάρμακον ἀντίδοτον ''(pharmakon antidoton)'', "(medicine) given as a remedy". An older term in English which is now rare is atterlothe, derived from " atter" ("poison" or "venom"). Antidotes for anticoagulants are sometimes referred to as reversal agents. The antidotes for some particular toxins are manufactured by injecting the toxin into an animal in small doses and extracting the resulting antibodies from the host animals' blood. This results in an antivenom that can be used to counteract venom produced by certain species of snakes, spiders, and other venomous animals. Some animal venoms, especially those produced by arthropods (such as certain spiders, scorpions, and bees) are only potentially lethal when they provoke allergic reactions and induce anaphylactic shock; as such, there is no "antidote" for these venoms; however anaphylactic shoc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nerve Agent
Nerve agents, sometimes also called nerve gases, are a class of organic chemistry, organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that catalyzes the breakdown of acetylcholine, a neurotransmitter. Nerve agents are irreversible acetylcholinesterase inhibitors used as poison. Poisoning by a nerve agent leads to constriction of pupils, profuse salivation, convulsions, and involuntary urination and defecation, with the first symptoms appearing in seconds after exposure. Death by asphyxiation or cardiac arrest may follow in minutes due to the loss of the body's control over Respiration (physiology), respiratory and other muscles. Some nerve agents are readily vaporized or aerosolized, and the primary portal of entry into the body is the respiratory system. Nervous agents can also be absorbed through the skin, requiring that those likely to be subjected to su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pralidoxime
Pralidoxime (2-pyridine aldoxime methyl chloride) or 2-PAM, usually as the chloride or iodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to treat organophosphate poisoning in conjunction with atropine and either diazepam or midazolam. It is a white solid. Chemical synthesis Pralidoxime, 2-pyridinaldoxime methylchloride, is prepared by treating pyridine-2-carboxaldehyde with hydroxylamine. The resulting pyridine-2-aldoxime is alkylated with methyl iodide giving pralidoxime as the iodide salt. Mechanism of action Pralidoxime is typically used in cases of organophosphate poisoning. Organophosphates such as sarin bind to the hydroxy component (the site) of the active site of the acetylcholinesterase enzyme, thereby blocking its activity. Pralidoxime binds to the other half (the unblocked, anionic site) of the active site and then displaces the phosphate from the serine residue. The conjoine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholinergic Crisis
A cholinergic crisis is an over-stimulation at a neuromuscular junction due to an excess of acetylcholine, as a result of the inactivity of the acetylcholinesterase enzyme, which normally breaks down acetylcholine. Signs and symptoms As a result of cholinergic crisis, the muscles stop responding to the high synaptic levels of acetylcholine, leading to flaccid paralysis, respiratory failure, and other signs and symptoms reminiscent of organophosphate poisoning. Other symptoms include increased sweating, salivation, bronchial secretions along with miosis (constricted pupils). Some of the symptoms of increased cholinergic stimulation include: * Salivation: stimulation of the salivary glands * Lacrimation: stimulation of the lacrimal glands (tearing) * Urination: relaxation of the internal sphincter muscle of urethra, and contraction of the detrusor muscles * Defecation * Gastrointestinal distress: Smooth muscle tone changes causing gastrointestinal problems, including cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylcholinesterase Inhibitors
Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from Hydrolysis, breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, Autonomic ganglion, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being Butyrylcholinesterase#Inhibitors, butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase, cholinesterase enzyme family. Acetylcholinesterase inhibitors are classified as reversible, irreversible, or quasi-irreversible (also called pseudo-irreversible). Mechanism of action Organophosphates Organophosphates like TEPP, tetraethyl pyrophosphate (TEPP) and sarin inhibit cholinesterases, enzymes that hydrolyze the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinic Acetylcholine Receptor
Nicotinic acetylcholine receptors, or nAChRs, are Receptor (biochemistry), receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the Sympathetic nervous system, sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atropine
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically given intravenously or by injection into a muscle. Eye drops are also available which are used to treat uveitis and early amblyopia. The intravenous solution usually begins working within a minute and lasts half an hour to an hour. Large doses may be required to treat some poisonings. Common side effects include dry mouth, abnormally large pupils, urinary retention, constipation, and a fast heart rate. It should generally not be used in people with closed-angle glaucoma. While there is no evidence that its use during pregnancy causes birth defects, this has not been well studied so sound clinical judgment should be used. It is likely safe during breastfeeding. It is an antimuscarinic (a type of anticholinergic) that works by inhibit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muscarinic Acetylcholine Receptor
Muscarinic acetylcholine receptors (mAChRs) are acetylcholine receptors that form G protein-coupled receptor, G protein-coupled receptor complexes in the cell membranes of certain neurons and other Cell (biology), cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers. They are mainly found in the parasympathetic nervous system, but also have a role in the sympathetic nervous system in the control of Sweat gland, sweat glands. Muscarinic receptors are so named because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpine and Hyoscine hydrobromide, scopolamine) manipulate these two distinct receptors by acting as selective agonists or receptor antagonist, antagonists. Function acetylcholine, Ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |