|
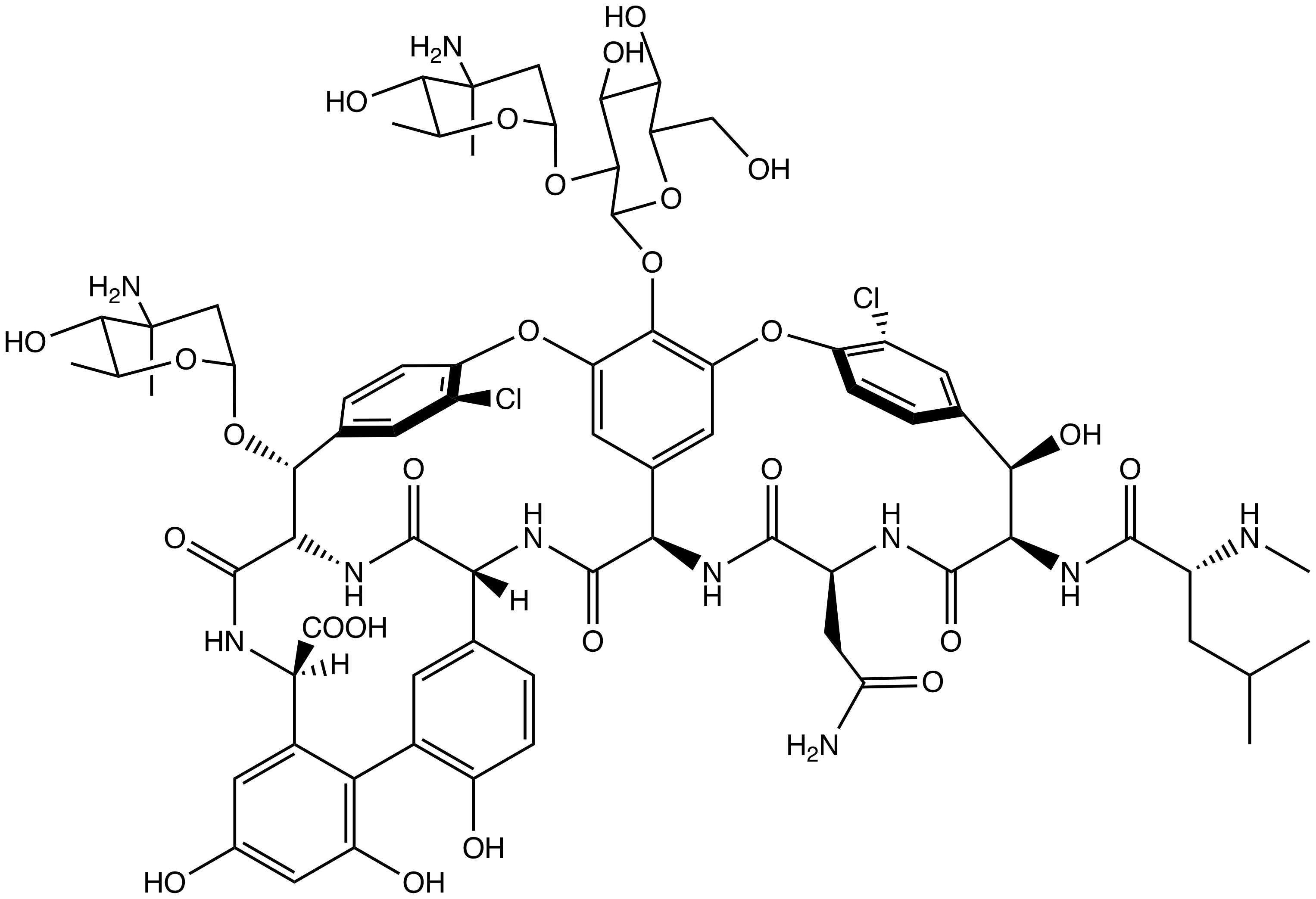
Chloroeremomycin
Chloroeremomycin is a member of the glycopeptide family of antibiotics, such as vancomycin. The molecule is a non-ribosomal polypeptide that has been glycosylated. It is composed of seven amino acids and three saccharide units. Although chloroeremomycin has never been used in human medicine, oritavancin, a semi-synthetic derivative of chloroeremomycin, has full FDA approval. Chloroeremomycin is a type of glycopeptide antibiotic and works by blocking the construction of a cell wall. Chloroeremomycin is naturally produced by ''Amycolatopsis orientalis''. History Chloroeremomycin was discovered by Eli Lilly in the 1980s. In the 1990s, researchers at Eli Lilly developed biphenyl-chloroeremomycin, now known as oritavancin, as a functionalized derivative of chloroeremomycin to combat rising antibacterial resistance to vancomycin. The chloroeremomycin gene cluster was sequenced by van Wageningen ''et al'' in 1998.van Wageningen, A. M. A., Kirkpatrick, P. N., Williams, D. H., Harris, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Hydroxyphenylglycine
4-Hydroxyphenylglycine (HPG) is a Non-proteinogenic amino acids, non-proteogenic amino acid found in vancomycin and related glycopeptides. HPG is synthesized from the Shikimate pathway, shikimic acid pathway and requires four enzymes to synthesize: Both L- and D-HPG are used in the vancomycin class of antibiotics. Tyrosine, a similar amino acid, differs by a methylene group (CH2) between the aromatic ring and the alpha carbon. Biosynthesis : HPG is synthesized from Prephenic acid, prephenate, an intermediate in the shikimic acid pathway and also a precursor to tyrosine. Prephenate is aromatized by prephenate dehydrogenase (Pdh) using Nicotinamide adenine dinucleotide, NAD+ as a cofactor to produce 4-hydroxyphenylpyruvate. 4-Hydroxyphenylpyruvate is then oxidized by 4-hydroxymandelate synthase (4HmaS) using oxygen to form 4-hydroxymandelate and hydrogen peroxide. 4HmaS is a non-heme iron-dependent dioxygenase. The reaction mechanism of this unique oxidation was proposed by Chorob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycopeptide Antibiotics
Glycopeptide antibiotics are a class of drugs of microbial origin that are composed of glycosylated cyclic or polycyclic nonribosomal peptides. Significant glycopeptide antibiotics include the anti-infective antibiotics vancomycin, teicoplanin, telavancin, ramoplanin, avoparcin and decaplanin, corbomycin, complestatin and the antitumor antibiotic bleomycin. Vancomycin is used if infection with methicillin-resistant Staphylococcus aureus, methicillin-resistant ''Staphylococcus aureus'' (MRSA) is suspected. Mechanism and classification Some members of this class of drugs inhibit the synthesis of cell walls in susceptible microbes by inhibiting peptidoglycan synthesis. The core class (including vancomycin) binds to acyl-D-alanyl-D-alanine in lipid II, preventing the addition of new units to the peptidoglycan. Of this core class, one may distinguish multiple generations: the first generation includes vancomycin and teicoplanin, while the semisynthetic second generation incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Teixobactin
Teixobactin () is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall. Teixobactin was discovered using a new method of culturing bacteria in soil, which allowed researchers to grow a previously unculturable bacterium now named '' Eleftheria terrae'', which produces the antibiotic. Teixobactin was shown to kill ''Staphylococcus aureus'' and ''Mycobacterium tuberculosis''. History In January 2015, a collaboration of four institutes in the US and Germany together with two pharmaceutical companies, reported that they had isolated and characterized a new antibiotic, killing "without detectable resistance." Teixobactin was discovered by screening previously unculturable bacteria present in a sample of soil from "a grassy field in Maine," using the isolation chip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycorandomization
Glycorandomization, is a drug discovery and drug development technology platform to enable the rapid diversification of bioactive small molecules, drug leads and/or approved drugs through the attachment of sugars. Initially developed as a facile method to manipulate carbohydrate substitutions of naturally occurring glycosides to afford the corresponding differentially glycosylated natural product libraries, glycorandomization applications have expanded to include both small molecules (drug leads and approved drugs) and even macromolecules (proteins). Also referred to as 'glycodiversification', glycorandomization has led to the discovery of new glycoside analogs which display improvements in potency, selectivity and/or ADMET as compared to the parent molecule. Classification The traditional method for attaching sugars to natural products, drugs or drug leads is by chemical glycosylation. This classical approach typically requires multiple protection/deprotection steps in additi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atropisomer
Atropisomers are stereoisomers arising because of hindered rotation about a covalent bond, single bond, where Gibbs free energy, energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual rotamers. They occur naturally and are of occasional importance in pharmaceutical design. When the substituents are achiral, these conformers are enantiomers (''atropoenantiomers''), showing axial chirality; otherwise they are diastereomers (''atropodiastereomers''). Etymology and history The word ''atropisomer'' (, , meaning "not to be turned") was coined in application to a theoretical concept by German biochemist Richard Kuhn for Karl Freudenberg's seminal ''Stereochemie'' volume in 1933. Atropisomerism was first experimentally detected in a tetra substituted biphenyl, a diacid, by George Christie and James Kenner in 1922. Michinori Ōki further refined the definition of atropisomers taking into account th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylation
Methylation, in the chemistry, chemical sciences, is the addition of a methyl group on a substrate (chemistry), substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen#Compounds, hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and biology. In biological systems, methylation is Catalysis, catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of Protein#Functions, protein function, and RNA processing. ''In vitro'' methylation of tissue samples is also a way to reduce some histology#Histological Artifacts, histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals and can regulate gene expression, RNA processing, and protein function. It is a key pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer Projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry. The use of Fischer projections in non-carbohydrates is discouraged, as such drawings are ambiguous and easily confused with other types of drawing. The main purpose of Fischer projections is to show the chirality of a molecule and to distinguish between a pair of enantiomers. Some notable uses include drawing sugars and depicting isomers. Conventions All bonds are depicted as horizontal or vertical lines. The carbon chain is depicted vertically, with carbon atoms sometimes not shown and represented by the center of crossing lines (see figure below). The orientation of the carbon chain is so that the first carbon (C1) is at the top. In an aldose, C1 is the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups creates a new stereoisomer. Stereocenters are also referred to as stereogenic centers. A stereocenter is geometrically defined as a point (location) in a molecule; a stereocenter is usually but not always a specific atom, often carbon. Stereocenters can exist on Chirality (chemistry), chiral or achiral molecules; stereocenters can contain single bonds or double bonds. The number of hypothetical stereoisomers can be predicted by using 2''n'', with ''n'' being the number of Tetrahedral molecular geometry, tetrahedral stereocenters; however, exceptions such as Meso compound, meso compounds can reduce the prediction to below the expected 2''n''. Chirality (chemistry), Chirality centers are a type of stereocenter with four different substituen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vancosamine
Vancosamines are aminosugars that are a part of vancomycin and other molecules within the vancomycin family of antibiotics. Vancosamine synthesis is encoded by the vancomycin (''vps'') biosynthetic cluster. Epivancosamine, a closely related aminosugar, is encoded by the chloroeremomycin (''cep'') biosynthetic cluster. History Vancosamine was first isolated by Lomakina ''et al'' in 1968. In 1972, Johnson ''et al'' were the first to identify and completely characterize vancosamine. Epivancosamine was subsequently isolated in 1988 by Hunt ''et al'' at Eli Lilly Biosynthesis The biosynthesis of vancosamine and epivancosamine are identical, except in the last step. The enzymes that catalyze the reactions have been designated EvaA-E. A molecule of TDP-D-glucose enters the pathway via conversion to molecule 1 by an oxidoreductase enzyme and then a dehydratase enzyme. In the next step, EvaA dehydrates molecule 1 by deprotonating at 3-C to form and enolate, which then eliminates 2-OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not always in chemistry), glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation (also 'non-enzymatic glycation' and 'non-enzymatic glycosylation') may refer to a non-enzymatic reaction. Glycosylation is a form of co-translational and post-translational modification. Glycans serve a variety of structural and functional roles in membrane and secreted proteins. The majority of proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as the ''O''-GlcNAc modification. Aglycosylation is a feature of engineered antibodies to bypass glycosylation. Five classes of glycans are produced: * ''N''-linked glycans attached to a nitrogen of asparagi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |