|
Chloride Cells
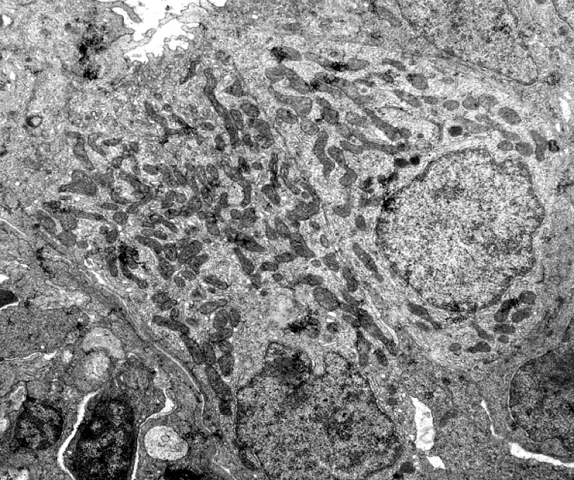
An ionocyte (formerly called a chloride cell) is a mitochondrion-rich cell within ionoregulatory organs of animals, such as teleost fish gill, insect Malpighian tubules, crustacean gills, antennal glands and maxillary glands, and copepod Crusalis organs. These cells contribute to the maintenance of optimal osmotic, ionic, and acid-base levels within metazoans. In aquatic invertebrates, ionocytes perform the functions of both ion uptake and ion excretion. In marine teleost fish, by expending energy to power the enzyme Na+/K+-ATPase and in coordination with other protein transporters, ionocytes pump excessive sodium and chloride ions against the concentration gradient into the ocean. Conversely, freshwater teleost ionocytes use this low intracellular environment to attain sodium and chloride ions into the organism, and also against the concentration gradient. In larval fishes with underdeveloped / developing gills, ionocytes can be found on the skin and fins. Mechanism of action M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride Cell
An ionocyte (formerly called a chloride cell) is a mitochondrion-rich cell within ionoregulatory organs of animals, such as teleost fish gill, insect Malpighian tubules, crustacean gills, antennal glands and maxillary glands, and copepod Crusalis organs. These cells contribute to the maintenance of optimal osmotic, ionic, and acid-base levels within metazoans. In aquatic invertebrates, ionocytes perform the functions of both ion uptake and ion excretion. In marine teleost fish, by expending energy to power the enzyme Na+/K+-ATPase and in coordination with other protein transporters, ionocytes pump excessive sodium and chloride ions against the concentration gradient into the ocean. Conversely, freshwater teleost ionocytes use this low intracellular environment to attain sodium and chloride ions into the organism, and also against the concentration gradient. In larval fishes with underdeveloped / developing gills, ionocytes can be found on the skin and fins. Mechanism of action ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondrion
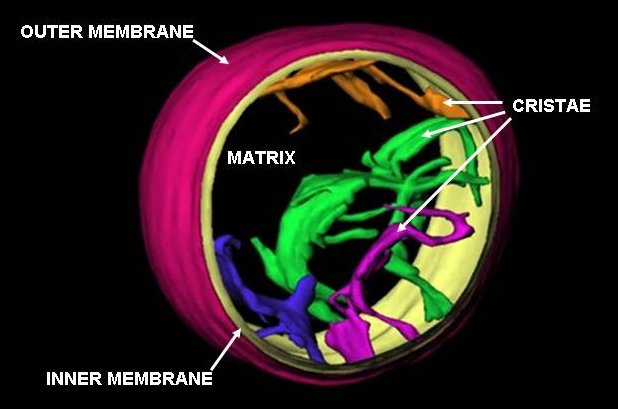
A mitochondrion () is an organelle found in the cell (biology), cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'', meaning a thread-like granule, was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 ''Scientific American'' article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). The multicellular animal ''Henneguya zschokkei, Henneguya salminicola'' is known to have retained mitochondrion-related organelles despite a complete loss of their mitochondrial genome. A large number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (biology)
The cell is the basic structural and functional unit of all life, forms of life. Every cell consists of cytoplasm enclosed within a Cell membrane, membrane; many cells contain organelles, each with a specific function. The term comes from the Latin word meaning 'small room'. Most cells are only visible under a light microscope, microscope. Cells Abiogenesis, emerged on Earth about 4 billion years ago. All cells are capable of Self-replication, replication, protein synthesis, and cell motility, motility. Cells are broadly categorized into two types: eukaryotic cells, which possess a Cell nucleus, nucleus, and prokaryotic, prokaryotic cells, which lack a nucleus but have a nucleoid region. Prokaryotes are single-celled organisms such as bacteria, whereas eukaryotes can be either single-celled, such as amoebae, or multicellular organism, multicellular, such as some algae, plants, animals, and fungi. Eukaryotic cells contain organelles including Mitochondrion, mitochondria, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Teleost
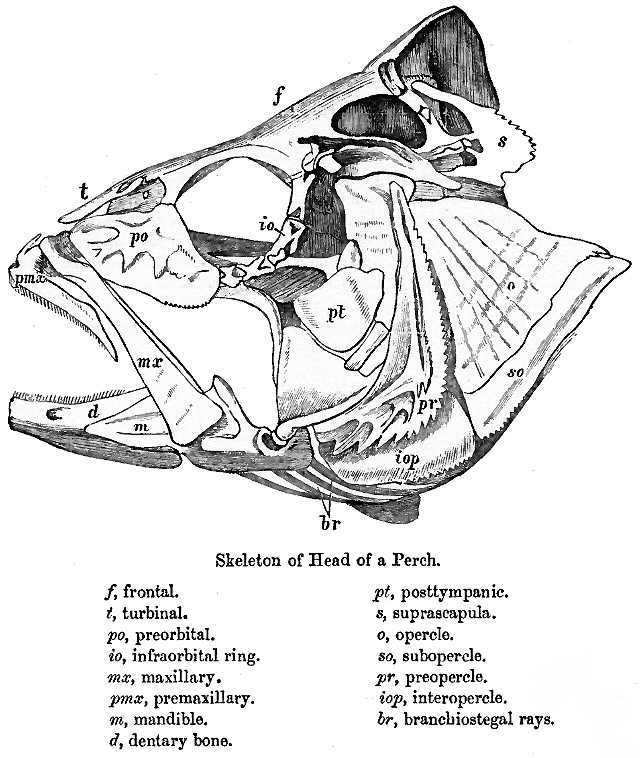
Teleostei (; Ancient Greek, Greek ''teleios'' "complete" + ''osteon'' "bone"), members of which are known as teleosts (), is, by far, the largest group of ray-finned fishes (class Actinopterygii), with 96% of all neontology, extant species of fish. The Teleostei, which is variously considered a Division (zoology), division or an infraclass in different taxonomic systems, include over 26,000 species that are arranged in about 40 order (biology), orders and 448 family (biology), families. Teleosts range from giant oarfish measuring or more, and ocean sunfish weighing over , to the minute male anglerfish ''Photocorynus spiniceps'', just long. Including not only torpedo-shaped fish built for speed, teleosts can be flattened vertically or horizontally, be elongated cylinders or take specialised shapes as in anglerfish and seahorses. The difference between teleosts and other bony fish lies mainly in their jaw bones; teleosts have a movable premaxilla and corresponding modifications ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fish Gill
Fish gills are Organ (anatomy), organs that allow fish to Aquatic respiration, breathe underwater. Most fish Respiration (physiology), exchange gases like oxygen and carbon dioxide using gills on both sides of the pharynx (throat). Gills possess Tissue (biology), tissues resembling short threads, referred to as gill filaments or Lamella (surface anatomy), lamellae. Each filament contains a capillary network that provides a large surface area for exchanging oxygen and carbon dioxide. Other than respiration, these filaments have other functions including the exchange of ions, water, acids, and ammonia. Fish respire by pulling oxygen-rich water through their mouths and pumping it over their gills. Within the gill filaments, capillary blood flows in the opposite direction to the water, causing countercurrent exchange. The gills push the oxygen-poor water out through openings in the sides of the pharynx. Some fish, like sharks and lampreys, possess multiple gill openings, but the mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the Abundance of elements in Earth's crust, sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been Leaching (chemistry), leached by the action of water from the Earth, Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in Soap, soap manufac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pronunciation of the word "chloride" is . Chloride salts such as sodium chloride are often soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Other examples of ionic chlorides include potassium chloride (), calcium chloride (), and ammonium chloride (). Examples of covalent chlorides include methyl chloride (), carbon tetrachloride (), sulfuryl chloride (), and monochloramine (). Electronic properties A chloride ion (diameter 167 pm) is much larger than a chlorine atom (diameter 99 pm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ (potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− (chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indicates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentration Gradient
Fick's laws of diffusion describe diffusion and were first posited by Adolf Fick in 1855 on the basis of largely experimental results. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation. ''Fick's first law'': Movement of particles from high to low concentration (diffusive flux) is directly proportional to the particle's concentration gradient. ''Fick's second law'': Prediction of change in concentration gradient with time due to diffusion. A diffusion process that obeys Fick's laws is called normal or Fickian diffusion; otherwise, it is called anomalous diffusion or non-Fickian diffusion. History In 1855, physiologist Adolf Fick first reported* * his now well-known laws governing the transport of mass through diffusive means. Fick's work was inspired by the earlier experiments of Thomas Graham, which fell short of proposing the fundamental laws for which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seawater
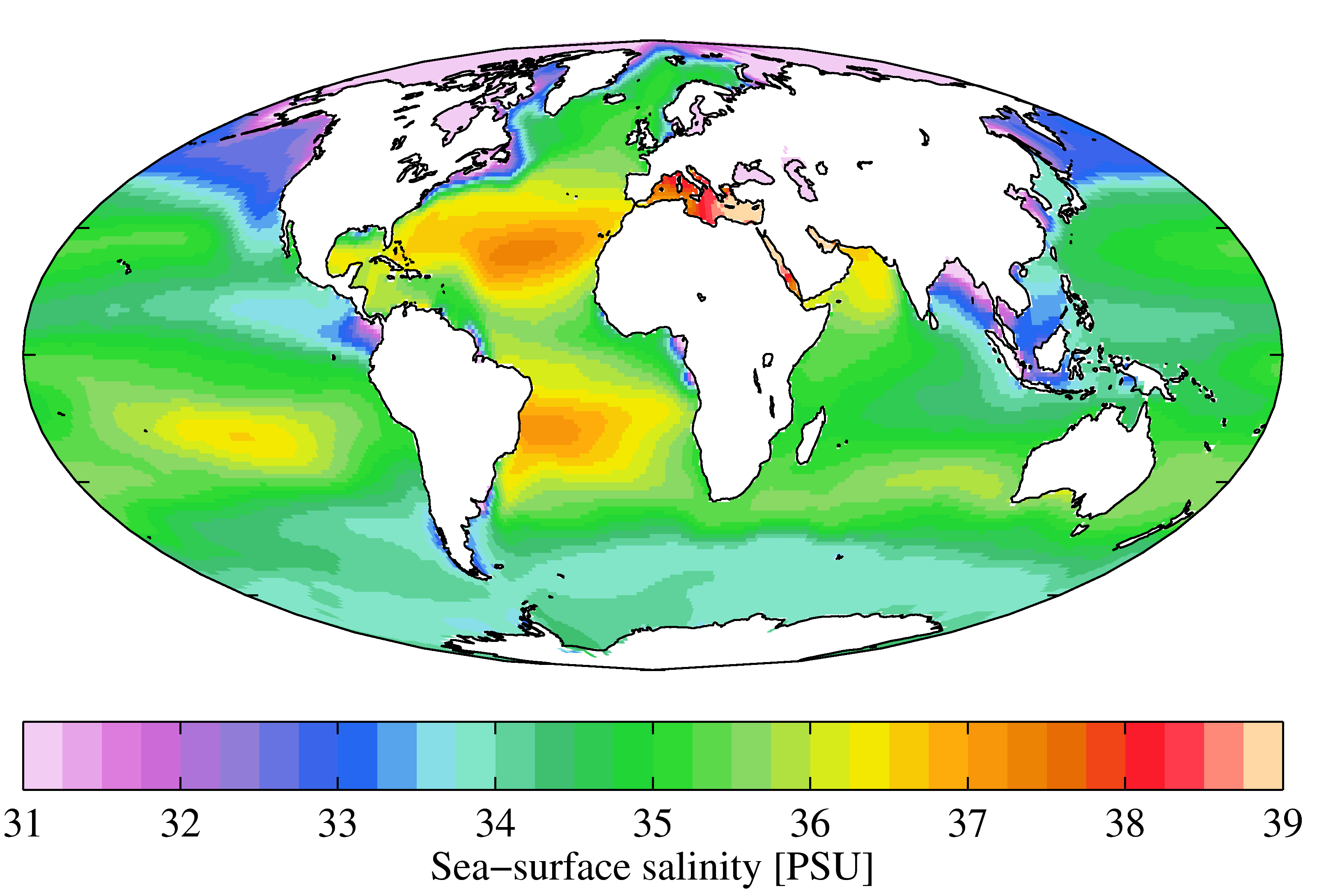
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately of dissolved salts (predominantly sodium () and chloride () ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at ) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about . The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was . Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measuremen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Dehydration
Osmotic dehydration is an operation used for the partial removal of water from plant tissues by immersion in a hypertonic (osmotic) solution. Sugar or salt solutions are used to reduce the moisture content of foods before actual drying process. This technique is used to give the product quality improvement over conventional drying process. Mild heat treatment after osmotic dehydration favours colour and flavour retention resulting in the product having superior organoleptic characteristics. It also increases resistance to heat treatment, prevents enzymatic browning and inhibits activities of polyphenol oxidase. The process is economical. Osmotic dehydration depends on: * Temperature of osmotic solution. * Concentration of the osmotic solution. * Osmotic agent used. * Process duration. * Geometry of food material. Process Water removal is based on the natural and non-destructive phenomenon of osmosis across cell membranes. The driving force for the diffusion of water from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |