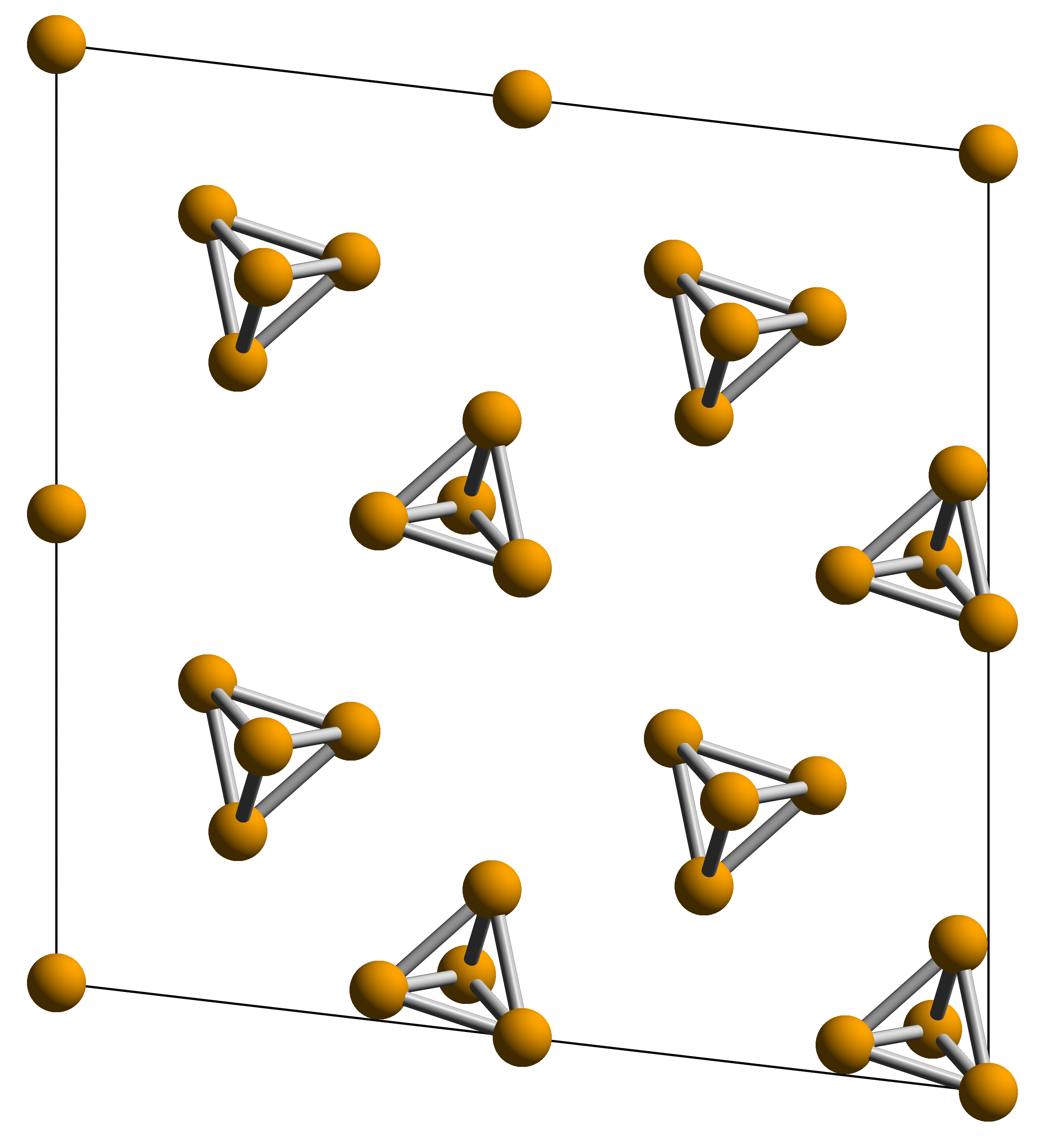|
Chemiluminescence Immunoassay
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction, i.e. a chemical reaction results in a flash or glow of light. A standard example of chemiluminescence in the laboratory setting is the luminol test. Here, blood is indicated by luminescence upon contact with iron in hemoglobin. When chemiluminescence takes place in living organisms, the phenomenon is called bioluminescence. A light stick emits light by chemiluminescence. Physical description As in many chemical reactions, chemiluminescence starts with the combining of two compounds, say A and B, to give a product C. Unlike most chemical reactions, the product C converts to a further product, which is produced in an electronically excited state often indicated with an asterisk: : A + B → C : C → D* D* then emits a photon (''h''ν), to give the ground state of D: I : D* → D + ''h''ν In theory, one photon of light should be given off for each mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed. In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emitti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
White Phosphorus
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phosphorus is for this reason called yellow phosphorus. White phosphorus is the first allotrope of phosphorus, and in fact the first elementary substance to be discovered that was not known since ancient times. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air. It is toxic, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic odor, and samples are commonly coated with white " diphosphorus pentoxide", which consists of tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ''electron donor''). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an Redox, oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word ''alkali'' is derived from Arabic ''al qalīy'' (or ''alkali''), meaning (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime''), a far more strongly basic substance known as ''caustic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luminol
Luminol (C8H7N3O2) is a chemical that exhibits chemiluminescence, with a blue glow, when mixed with an appropriate oxidizing agent. Luminol is a white-to-pale-yellow Crystal, crystalline solid that is soluble in most polar organic solvents but insoluble in water. Forensic investigators use luminol to detect trace amounts of blood at crime scenes, as it reacts with the iron in hemoglobin. Biologists use it in cellular assays to detect copper, iron, and cyanides as well as specific proteins via western blotting. When luminol is sprayed evenly across an area, trace amounts of an activating oxidant make the luminol emit a blue glow that can be seen in a darkened room. The glow only lasts about 30 seconds but can be documented photographically. The glow is stronger in areas receiving more spray; the intensity of the glow does not indicate the amount of blood or other activator present. Synthesis Luminol is synthesized in a two-step process, beginning with 3-nitrophthalic acid. Fir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemiluminescence
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction, i.e. a chemical reaction results in a flash or glow of light. A standard example of chemiluminescence in the laboratory setting is the luminol test. Here, blood is indicated by luminescence upon contact with iron in hemoglobin. When chemiluminescence takes place in living organisms, the phenomenon is called bioluminescence. A light stick emits light by chemiluminescence. Physical description As in many chemical reactions, chemiluminescence starts with the combining of two compounds, say A and B, to give a product C. Unlike most chemical reactions, the product C converts to a further product, which is produced in an electronically excited state often indicated with an asterisk: : A + B → C : C → D* D* then emits a photon (''h''ν), to give the ground state of D: I : D* → D + ''h''ν In theory, one photon of light should be given off for each mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dioxetane
A dioxetane or dioxacyclobutane is an organic compound with formula C2O2H4, whose backbone is a four-membered ring of two oxygen atoms and two carbon atoms. There are two isomers: * 1,2-dioxetane where the oxygen atoms are adjacent. * 1,3-dioxetane 1,3-Dioxetane (1,3-dioxacyclobutane) is a heterocyclic organic compound with formula C2O2H4, whose backbone is a four-member ring of alternating oxygen and carbon atoms. It can be viewed as a dimer of formaldehyde Formaldehyde ( , ) (system ... where the oxygen and carbon atoms alternate. {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lophine
Lophine is the organic compound with the formula . It is a derivative of imidazole wherein all three carbon atoms have phenyl groups as substituents. A white solid, this compound gave the first example of chemiluminescence when its basic solutions were exposed to air. Its chemiluminescence continues to attract attention. Lophine and its dihydro analogue amarine (''meso''-2,4,5-triphenyl-2-imidazoline) were discovered early in the history of organic chemistry (between 1841 and 1847), before the development of a structural theory of organic chemistry by Kekulé and Couper in the 1850s. Lophine is prepared by condensation of benzaldehyde, benzil, and ammonia Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ....{{cite journal , doi=10.1002/jhet.4771 , title=Catalyst-free one-pot sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lampyris Noctiluca (firefly) Mating
''Lampyris'' is a genus of beetles in the Lampyridae. In most of western Eurasia, they are the predominant members of this family and includes the European common glow-worm, which is the type species. They produce a continuous glow;Stanger-Hall, Kathrin F.; Lloyd, James E. & Hillis, David M. (2007): Phylogeny of North American fireflies (Coleoptera: Lampyridae): Implications for the evolution of light signals. '' Mol. Phylogenet. Evol.'' 45(1): 33-49. PDF fulltext the larvae and larviform females are among those organisms commonly called "glowworms". This genus is rather close to '' Pleotomus'' and its relatives. These were formerly separated as tribe Pleotomini, but appear to be a specialized offshoot of the Lampyrini. Species ''BioLib'' includes the following species:https://www.biolib.cz/en/taxon/id9567/ BioLib.cz: genus ''Lampyris'' Geoffroy, 1762 (retrieved 25 June 2020) * '' Lampyris algerica'' Ancey, 1869 * '' Lampyris ambigena'' Jacquelin du Val, 1860 * '' Lampyris angus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |