|
Chemical Specificity
Chemical specificity is the ability of binding site of a macromolecule (such as a protein) to bind specific ligands. The fewer ligands a protein can bind, the greater its specificity. Specificity describes the strength of binding between a given protein and ligand. This relationship can be described by a dissociation constant, which characterizes the balance between bound and unbound states for the protein-ligand system. In the context of a single enzyme and a pair of binding molecules, the two ligands can be compared as stronger or weaker ligands (for the enzyme) on the basis of their dissociation constants. (A lower value corresponds to a stronger binding.) Specificity for a set of ligands is unrelated to the ability of an enzyme to catalyze a given reaction, with the ligand as a substrate. If a given enzyme has a high chemical specificity, this means that the set of ligands to which it binds is limited, such that neither binding events nor catalysis can occur at an apprecia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins (resulting in a protein–protein interaction), enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent), but can also be covalent reversible or irreversible. Function Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts of signal transduction pathways. Types of ligands include neurotransmitters, toxins, neuropeptides, and steroid hormones. Binding site ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic Interactions
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and to be excluded by water. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar substances, which maximizes the entropy of water and minimizes the area of contact between water and nonpolar molecules. In terms of thermodynamics, the hydrophobic effect is the free energy change of water surrounding a solute. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Hence the hydrophobic effect is e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substrate (chemistry)
In chemistry, the term substrate is highly context-dependent. Broadly speaking, it can refer either to a chemical species being observed in a chemical reaction, or to a surface on which other chemical reactions or microscopy are performed. In the former sense, a reagent is added to the ''substrate'' to generate a product through a chemical reaction. The term is used in a similar sense in synthetic and organic chemistry, where the substrate is the chemical of interest that is being modified. In biochemistry, an enzyme substrate is the material upon which an enzyme acts. When referring to Le Chatelier's principle, the substrate is the reagent whose concentration is changed. ;Spontaneous reaction : :*Where S is substrate and P is product. ;Catalysed reaction : :*Where S is substrate, P is product and C is catalyst. In the latter sense, it may refer to a surface on which other chemical reactions are performed or play a supporting role in a variety of spectroscopic and micr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, including those that cause disease. Each individual antibody recognizes one or more specific antigens, and antigens of virtually any size and chemical composition can be recognized. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each of the branching chains comprising the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" the antigen (or a microbe or an infected cell bearing such an antigen) for attack by cells of the immune system, or can neutralize it directly (for example, by blocking a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ENZYME STRUCTURE AND FUNCTION
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include catalytic RNA molecules, also called ribozymes. They are sometimes described as a ''type'' of enzyme rather than being ''like'' an enzyme, but even in the dec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specificity Constant
In the field of biochemistry, the specificity constant (also called kinetic efficiency or k_/K_), is a measure of how efficiently an enzyme converts substrates into products. A comparison of specificity constants can also be used as a measure of the preference of an enzyme for different substrates (i.e., substrate specificity). The higher the specificity constant, the more the enzyme "prefers" that substrate. The following equation, known as the Michaelis–Menten model, is used to describe the kinetics of enzymes: : + S _fk_r] ES -> _ + P where E, S, ES, and P represent enzyme, substrate, enzyme–substrate complex, and product, respectively. The symbols k_f, k_r, and k_\mathrm denote the rate constants for the "forward" binding and "reverse" unbinding of substrate, and for the "catalytic" conversion of substrate into product, respectively. The Michaelis constant in turn is defined as follows: : K_ = \frac The Michaelis constant is equal to the substrate concentration a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michaelis Menten Equation
Michaelis or Michelis is a surname. Notable people and characters with the surname include: * Adolf Michaelis, German classical scholar * Alice Michaelis, German painter * Anthony R. Michaelis, German science writer * Christian Friedrich Michaelis, German doctor * Christian Friedrich Michaelis (philosopher), German essayist and philosopher * Edward Michelis, German theologian * Georg Michaelis, German politician * Gustav Adolf Michaelis, German obstetrician and namesake of the rhombus of Michaelis * Hans-Thorald Michaelis, German historian * Johann David Michaelis, German biblical scholar * John H. Michaelis, American four-star general * Laura Michaelis, American linguist * Leo Michelis, Greek-Canadian economist * Leonor Michaelis, German scientist known for Michaelis–Menten kinetics * Max Michaelis, South African financier * Margaret Michaelis-Sachs, Austrian-Australian photographer * Paul Charles Michaelis Paul Charles Michaelis was a Bell Labs researcher in magne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amylase
An amylase () is an enzyme that catalysis, catalyses the hydrolysis of starch (Latin ') into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of starch but little sugar, such as rice and potatoes, may acquire a slightly sweet taste as they are chewed because amylase degrades some of their starch into sugar. The pancreas and salivary gland make amylase (alpha amylase) to hydrolyse dietary starch into disaccharides and trisaccharides which are converted by other enzymes to glucose to supply the body with energy. Plants and some bacteria also produce amylase. Specific amylase proteins are designated by different Greek letters. All amylases are glycoside hydrolases and act on α-1,4-glycosidic bonds. Classification α-Amylase The α-amylases () (CAS registry number, CAS 9014–71–5) (alternative names: 1,4-α-D-glucan glucanohydrolase; glycogenase) are calcium metallop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
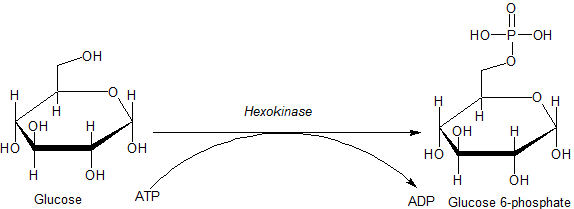
Hexokinase
A hexokinase is an enzyme that irreversibly phosphorylates hexoses (six-carbon sugars), forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexokinase possesses the ability to transfer an inorganic phosphate group from ATP to a substrate. Hexokinases should not be confused with glucokinase, which is a specific hexokinase found in the liver. All hexokinases are capable of phosphorylating several hexoses but hexokinase IV(D) is often misleadingly called glucokinase, though it is no more specific for glucose than the other mammalian isoenzymes. Variation Genes that encode hexokinase have been discovered in every domain of life, and exist among a variety of species that range from bacteria, yeast, and plants to humans and other vertebrates. The enzymes from yeast, plants and vertebrates all show clear sequence evidence of homology, but those of bacteria may not be relat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucokinase
Glucokinase () is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase is expressed in cells of the liver and pancreas of humans and most other vertebrates. In each of these organs it plays an important role in the regulation of carbohydrate metabolism by acting as a glucose sensor, triggering shifts in metabolism or cell function in response to rising or falling levels of glucose, such as occur after a meal or when fasting. Mutations of the gene for this enzyme can cause unusual forms of diabetes or hypoglycemia. Glucokinase (GK) is a hexokinase isozyme, related homologously to at least three other hexokinases. All of the hexokinases can mediate phosphorylation of glucose to glucose-6-phosphate (G6P), which is the first step of both glycogen synthesis and glycolysis. However, glucokinase is coded by a separate gene and its distinctive kinetic properties allow it to serve a different set of functions. Glucokinase has a lower affinity for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |