|
Carbyne
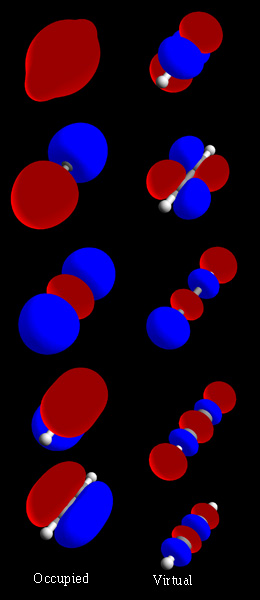
In organic chemistry, a carbyne is a general term for any compound whose structure consists of an electrically neutral carbon atom connected by a single covalent bond and has three non-bonded electrons. The carbon atom has either one or three unpaired electrons, depending on its excitation state; making it a radical. The chemical formula can be written or (also written as ), or just CH. Carbynes can be seen as derivatives of the simplest such compound, the methylidyne radical or unsubstituted carbyne or , in which the functional group is a hydrogen atom. Reported for the first time back in 1967 by Kasatochkin, carbyne is an infinite sp1 hybridized long linear chain of carbon, where each link is just a single carbon atom. Electronic configuration Carbyne molecules are generally found to be in electronic doublet states: the non-bonding electrons on carbon are arranged as one radical (unpaired electron) and one electron pair, leaving a vacant atomic orbital, rather than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Methylidyne Radical
Methylidyne, or (unsubstituted) carbyne, is an organic compound whose molecule consists of a single hydrogen atom bonded to a carbon atom. It is the parent compound of the carbynes, which can be seen as obtained from it by substitution of other functional groups for the hydrogen. The carbon atom is left with either one or three unpaired electrons (unsatisfied valence bonds), depending on the molecule's excitation state; making it a radical. Accordingly, the chemical formula can be CH• or CH3• (also written as ⫶CH); each dot representing an unpaired electron. The corresponding systematic names are methylidyne or hydridocarbon(•), and methanetriyl or hydridocarbon(3•). However, the formula is often written simply as CH. Methylidyne is a highly reactive gas that is quickly destroyed in ordinary conditions but is abundant in the interstellar medium (and was one of the first molecules to be detected there). Nomenclature The trivial name ''carbyne'' is the prefer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fluoromethylidyne
Fluoromethylidyne is not a stable chemical species but a metastable radical containing one highly reactive carbon atom bound to one fluorine atom with the formula CF. The carbon atom has a lone-pair and a single unpaired (radical) electron in the ground state. Ground-state fluoromethylidyne radicals can be produced by the ultraviolet photodissociation of dibromodifluoromethane at 248 nanometer wavelength. It readily and irreversibly dimerises to difluoroacetylene, also known as difluoroethyne, perfluoroacetylene, or di- or perfluoroethylyne. Under certain conditions it can hexamerise to hexafluorobenzene. See also * Carbyne In organic chemistry, a carbyne is a general term for any compound whose structure consists of an electrically neutral carbon atom connected by a single covalent bond and has three non-bonded electrons. The carbon atom has either one or three ... References Free radicals Reactive intermediates {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene radical, methylene, the parent hydride from which all other carbene compounds are formally derived. There are two types of carbenes: singlet state, singlets or triplet state, triplets, depending upon their electronic structure. The different classes undergo different reactions. Most carbenes are extremely reactive and short-lived. A small number (the diHalogen, halocarbenes, carbon monoxide, and carbon monosulfide) can be isolated, and can stabilize as Coordination complex, metal ligands, but otherwise cannot be stored in bulk. A rare exception are the persistent carbenes, which have extensive application in modern organometallic chemistry. Generatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reactive Intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation. When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place. Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, hence unstable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Electron Configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is , meaning that the 1s, 2s, and 2p subshells are occupied by two, two, and six electrons, respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by the nuclei and all the other electrons. Mathematically, configurations are described by Slater determinants or configuration state functions. According to the laws of quantum mechanics, a level of energy is associated with each electron configuration. In certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Molecular Orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms ''atomic orbital'' and ''molecular orbital'' were introduced by Robert S. Mulliken in 1932 to mean ''one-electron orbital wave functions''. At an elementary level, they are used to describe the ''region'' of space in which a function has a significant amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into a molecule by forming a valence chemical bond, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Orbital Overlap
In chemical bonds, an orbital overlap is the concentration of orbitals on adjacent atoms in the same regions of space. Orbital overlap can lead to bond formation. The general principle for orbital overlap is that, the greater the overlap between orbitals, the greater the bond strength. Linus Pauling explained the importance of orbital overlap in the molecular bond angles observed through experimentation; it is the basis for orbital hybridization. As ''s'' orbitals are spherical (and have no directionality) and ''p'' orbitals are oriented 90° to each other, a theory was needed to explain why molecules such as methane (CH4) had observed bond angles of 109.5°. Pauling proposed that s and p orbitals on the carbon atom can combine to form hybrids (sp3 in the case of methane) which are directed toward the hydrogen atoms. The carbon hybrid orbitals have greater overlap with the hydrogen orbitals, and can therefore form stronger C–H bonds.Pauling, Linus. (1960). ''The Nature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbine
A carbine ( or ) is a long gun that has a barrel shortened from its original length. Most modern carbines are rifles that are compact versions of a longer rifle or are rifles chambered for less powerful cartridges. The smaller size and lighter weight of carbines make them easier to handle. They are typically issued to high-mobility troops such as special operations soldiers and paratroopers, as well as to mounted, artillery, logistics, or other non-infantry personnel whose roles do not require full-sized rifles, although there is a growing tendency for carbines to be issued to front-line soldiers to offset the increasing weight of other issued equipment. An example of this is the M4 carbine, the standard issue carbine of the United States Armed Forces. Etymology The name comes from its first users — cavalry troopers called " carabiniers", from the French ''carabine'', from Old French ''carabin'' (soldier armed with a musket), whose origin is unclear. One theory connects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Non-bonding Orbital
A non-bonding orbital, also known as ''non-bonding molecular orbital'' (NBMO), is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms. Non-bonding orbitals are often designated by the letter n in molecular orbital diagrams and electron transition notations. Non-bonding orbitals are the equivalent in molecular orbital theory of the lone pairs in Lewis structures. The energy level of a non-bonding orbital is typically in between the lower energy of a valence shell bonding orbital and the higher energy of a corresponding antibonding orbital. As such, a non-bonding orbital with electrons would commonly be a HOMO ( highest occupied molecular orbital). According to molecular orbital theory, molecular orbitals are often modeled by the linear combination of atomic orbitals. In a simple diatomic molecule such as hydrogen fluoride (chemical formula: HF), one atom may have many more electrons than the other. A sigm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the structure. For example, , , and are isoelectronic, while and = are not. This definition is sometimes termed valence isoelectronicity. Definitions can sometimes be not as strict, sometimes requiring identity of the total electron count and with it the entire electronic configuration. More usually, definitions are broader, and may extend to allowing different numbers of atoms in the species being compared.A. A. Aradi & T. P. Fehlner, "Isoelectronic Organometallic Molecules", in F. G. A. Stone & Robert West (eds.) ''Advances in Organometallic Chemistry Vol. 30'' (1990), Chapter 5 (at p. 190google books link/ref> The importance of the concept lies in identifying significantly related species, as pairs or series. Isoelectronic specie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |