|
Carboxylate Complex
Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted intense scholarly scrutiny. Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging. Acetate and related monocarboxylates Structure and bonding Carboxylates bind to single metals by one or both oxygen atoms, the respective notation being κ1- and κ2-. In terms of electron counting, κ1-carboxylates are "X"-type ligands, i.e., a pseudohalide-like. κ2-carboxylates are "L-X ligands", i.e. resembling the combination of a Lewis base (L) and a pseudohalide (X). Carboxylates are classified as hard ligands, in HSAB theory. File:BasicFeacetate.png, Basic ferric acetate File:Ag2(OAc)2.png, Silver acetate File:BasicZnAcetate.png, Basic zinc acetate File:Mo2(OAc)4.svg, Moly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II)-acetate-tetrahydrate-3D-balls
Nickel is a chemical element; it has Chemical symbol, symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and Ductility, ductile transition metal. Pure nickel is chemically reactive, but large pieces are slow to react with air under Standard temperature and pressure, standard conditions because a Passivation (chemistry), passivation layer of Nickel(II) oxide, nickel oxide forms on the surface that prevents further corrosion. Even so, pure native metal, native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger iron meteorite, nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric iron, Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer core, Earth's outer a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chinese Lantern Structure
In chemistry, the Chinese lantern structure is a coordination complex where two metal atoms are bridged by four Denticity, bidentate ligands. This structure type is also known as a paddlewheel complex. Examples include chromium(II) acetate, molybdenum(II) acetate, and rhodium(II) acetate, copper(II) acetate dihydrate. The name is derived from the resemblance between the structure and a Chinese paper lantern. Often additional ligands are bound to the metal centers along the M---M vector. The degree of metal-metal bonding varies according to the d-electron configuration. Complexes with Chinese lantern structure *Copper benzoate *Copper acetate *Chromium(II) acetate *Molybdenum(II) acetate *Diruthenium tetraacetate chloride *Rhodium acetate References Further reading * Coordination chemistry {{inorganic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concerted Metalation Deprotonation
Concerted metalation-deprotonation (CMD) is a mechanistic pathway through which transition-metal catalyzed Carbon–hydrogen bond activation, C–H activation reactions can take place. In a CMD pathway, the C–H bond of the substrate is cleaved and the new C–Metal bond forms through a single transition state. This process does not go through a metal hydride species that is bound to the cleaved hydrogen atom. Instead, a carboxylate or carbonate base Deprotonation, deprotonates the substrate. The first proposal of a concerted metalation deprotonation pathway was by S. Winstein and T. G. Traylor in 1955 for the acetolysis of diphenylmercury. It was found to be the lowest energy transition state in a number of computational studies, was experimentally confirmed through Nuclear magnetic resonance, NMR experiments, and has been hypothesized to occur in mechanistic studies. While there are a number of different possible mechanisms for C–H activation, a CMD pathway is common for high va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimolybdenum Tetraacetate
Molybdenum(II) acetate is a coordination compound with the formula Mo2(O2CCH3)4. It is a yellow, diamagnetic, air-stable solid that is slightly soluble in organic solvents. Molybdenum(II) acetate is an iconic example of a compound with a metal-metal quadruple bond.Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J., "Synthesis and Technique in Inorganic Chemistry third edition", University Science Books: Mill Valley, CA, 1999, Structure Like several other transition metal carboxylate complexes, Mo2(O2CCH3)4 adopts a Chinese lantern structure. Each Mo(II) center in Mo2(O2CCH3)4 has four d valence electrons. These eight d-electrons form one σ, two π bonds, and one δ bond, creating a bonding electron configuration of σ2π4δ2. Each of these bonds are formed by the overlapping of pairs of d orbitals.Blaudeau, J. P.; Pitzer, R. M. “ Ab Initio Studies of Ligand Effects on the Metal-Metal Bond in Dimolybdenum Complexes” J.Phys. Chem. 1994, vol. 98, pp. 4575-4579. The four ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octachlorodimolybdate
Potassium octachlorodimolybdate (systematically named potassium bis(tetrachloromolybdate)(''Mo''–''Mo'')(4−)) is an inorganic compound with the chemical formula . It is known as a red-coloured, microcrystalline solid. The anion is of historic interest as one of the earliest illustrations of a quadruple bonding. The salt is usually obtained as the pink-coloured dihydrate. The compound is prepared in two steps from molybdenum hexacarbonyl: : : The reaction of the acetate with HCl was first described as providing trimolybdenum compounds, but subsequent crystallographic analysis confirmed that the salt contains the anion, with D4h symmetry, in which the two Mo atoms are linked by a quadruple bond. Each Mo atom is bounded with four ligands by a single bond. Each group is a regular square pyramid, with an Mo atom at the apex, and four Cl atoms at the vertices of the square base of the pyramid A pyramid () is a structure whose visible surfaces are triangular in broad o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
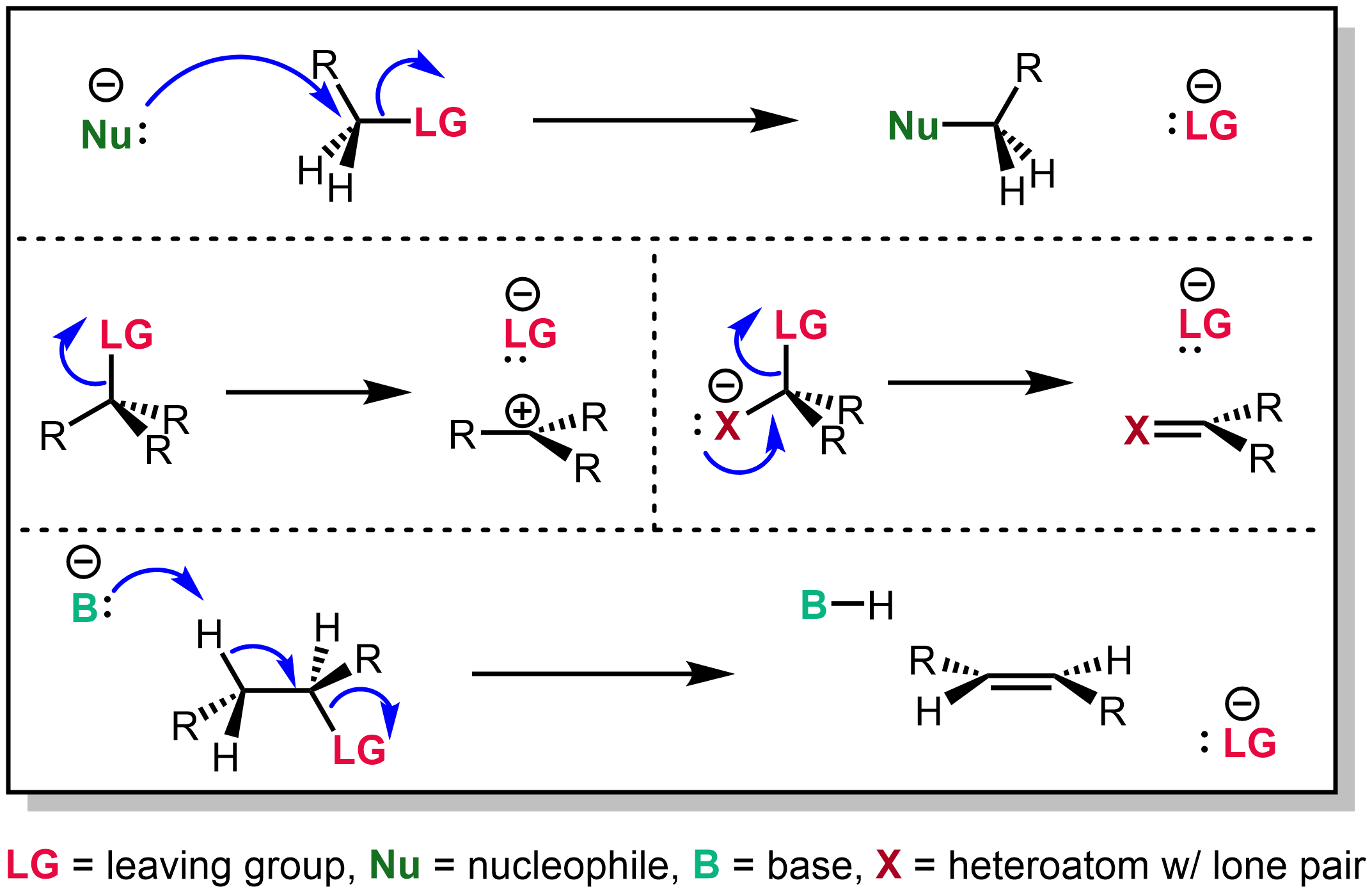
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt Metathesis Reaction
A salt metathesis reaction (also called a double displacement reaction, double replacement reaction, or double decomposition) is a type of chemical reaction in which two ionic compounds in aqueous solution exchange their component ions to form two new compounds. Often, one of these new compounds is a precipitate, gas, or weak electrolyte, driving the reaction forward. :AB + CD -> AD + CB \mathitA_\mathitD_\mathit + \mathitC_\mathitB_\mathit --> In older literature, the term double decomposition is common. The term double decomposition is more specifically used when at least one of the substances does not dissolve in the solvent, as the ligand or ion exchange takes place in the solid state of the reactant. For example: :AX(aq) + BY(s) → AY(aq) + BX(s). Types of reactions Counterion exchange Salt metathesis is a common technique for exchanging counterions. The choice of reactants is guided by a solubility chart or lattice energy. HSAB theory can also be used to predict ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination. Role in transition metal chemistry For transition metals, oxidative reaction results in the decrease in the d''n'' to a configuration with fewer electrons, often 2e fewer. Oxidative addition is favored for metals that are (i) basic and/or (ii) easily oxidized. Metals with a relatively low oxidation state often satisfy one of these requirements, but even high oxidation state metals undergo oxidative addition, as illustrated by the oxidation of Pt(II) with chlorine: : tCl4sup>2− + Cl2 → tCl6sup>2− In classical organometallic chemistry, the formal oxidation state of the metal and the electron count of the complex both in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Adamantanecarboxylic Acid
1-Adamantanecarboxylic acid is an organic compound with the formula . A white solid, it is the simplest carboxylic acid derivative of adamantane Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the mo .... The compound is notable for its synthesis by carboxylation of adamantane. 1-Adamantanecarboxylic acid is unusual in forming mononuclear tris(carboxylate) coordination complexes of the formula (O2CR)3sup>− (M = Mn, Ni, Co, Zn). See also * 1,3,5,7-Adamantanetetracarboxylic acid References {{DEFAULTSORT:Adamantanecarboxylic acid, 1- Adamantanecarboxylic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium(II) Acetate
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form together a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum(II) Acetate
Platinum(II) acetate is a purple-colored coordination complex. The complex adopts an unusual structure consisting of a square array of Pt atoms. Preparation Several syntheses of platinum(II) acetate have been reported. Geoffrey Wilkinson ''et al.'' reported a highly temperamental synthesis from sodium hexahydroxyplatinate, nitric acid, and acetic acid. This intermediate solution was reducted with formic acid. Alternatively, the complex can be prepared by the reaction of silver acetate with platinum(II) chloride. Structure According to X-ray crystallography X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ..., the complex is tetrameric, in contrast to the trimeric palladium analog. The four platinum atoms form a square cluster, with eight bridging acetate ligands surrounding th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(II) Acetate
Chromium(II) acetate hydrate, also known as chromous acetate, is the coordination compound with the chemical formula, formula Cr2(CH3CO2)4(H2O)2. This formula is commonly abbreviated Cr2(OAc)4(H2O)2. This red-coloured compound features a quadruple bond. It exists as the dihydrate and the anhydrous forms. Both are diamagnetic. Cr2(OAc)4(H2O)2 is a reddish diamagnetic powder, although diamond-shaped tabular crystals can be grown. Consistent with the fact that it is nonionic, Cr2(OAc)4(H2O)2 exhibits poor solubility in water and methanol. Structure The Cr2(OAc)4(H2O)2 molecule contains two atoms of chromium, two ligand, ligated molecules of water, and four acetate bridging ligands. The coordination environment around each chromium atom consists of four oxygen atoms (one from each acetate ligand) in a square, one water molecule (in an axial position), and the other chromium atom (opposite the water molecule), giving each chromium centre an Octahedral molecular geometry, octahedral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |