|
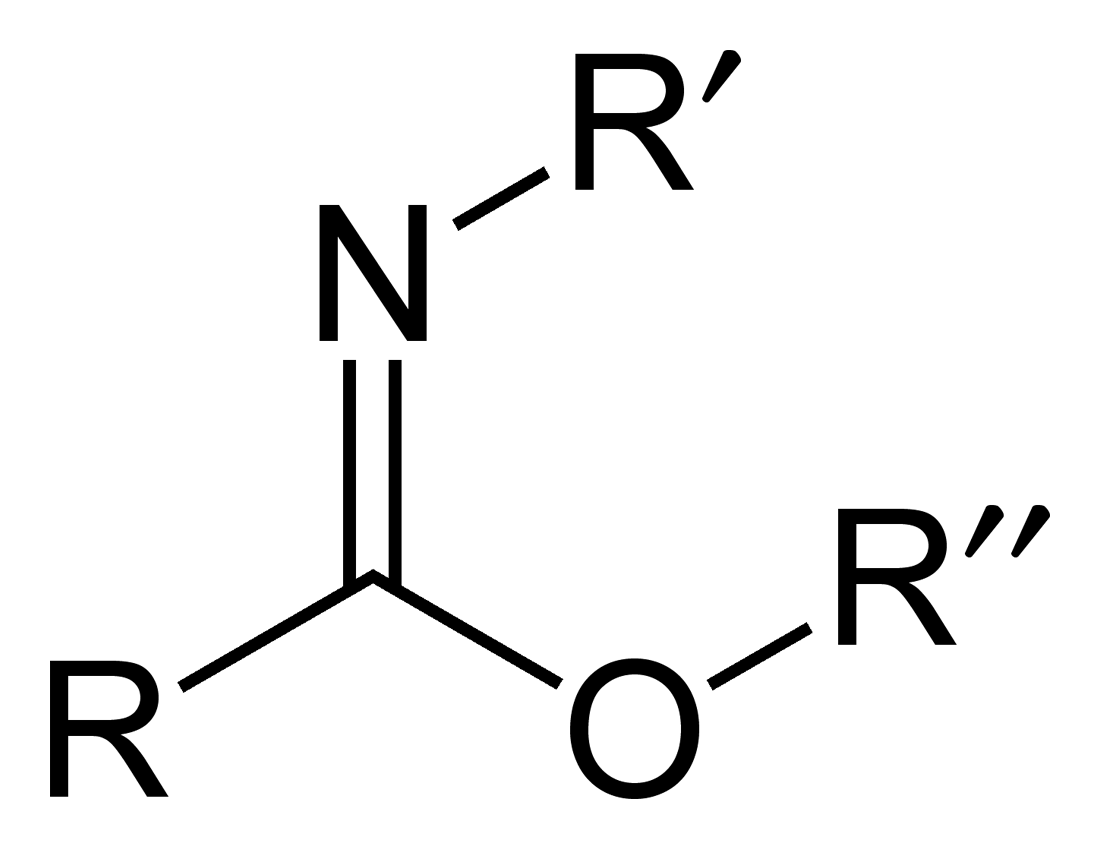
Carboximidate
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between a carboximidic acid (R-C(=NR')OH) and an alcohol, with the general formula R-C(=NR')OR". They are also known as imino ethers, since they resemble imines (>C=N-) with an oxygen atom connected to the carbon atom of the C=N double bond. Synthesis Imidates may be generated by a number of synthetic routes, but are in general formed by the Pinner reaction. This proceeds via the acid catalyzed attack of nitriles by alcohols. Imidates produced in this manner are formed as their hydrochloride salts, which are sometimes referred to as Pinner salts. Carboximidates are also formed as intermediates in the Mumm rearrangement and the Overman rearrangement. Imidate/amidate anions An amidate/imidate anion is formed upon deprotonation of an amide or imidic acid. Since amides and imidic acids are tautomers, they form the same anion upon deprotonation. The two names are thus s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chapman Rearrangement
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between a imidic acid, carboximidic acid (R-C(=NR')OH) and an Alcohol (chemistry), alcohol, with the general formula R-C(=NR')OR". They are also known as imino ethers, since they resemble imines (>C=N-) with an oxygen atom connected to the carbon atom of the C=N double bond. Synthesis Imidates may be generated by a number of synthetic routes, but are in general formed by the Pinner reaction. This proceeds via the acid catalyzed attack of nitriles by alcohols. Imidates produced in this manner are formed as their hydrochloride salts, which are sometimes referred to as Pinner salts. Carboximidates are also formed as intermediates in the Mumm rearrangement and the Overman rearrangement. Imidate/amidate anions An amidate/imidate anion is formed upon deprotonation of an amide or imidic acid. Since amides and imidic acids are tautomers, they form the same anion upon deprotonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mumm Rearrangement
The Mumm rearrangement is an organic reaction and a rearrangement reaction. It describes a 1,3(O-N) acyl transfer of an acyl imidate or isoimide group to an imide. The reaction is of relevance as part of the Ugi reaction The Ugi reaction is a multi-component reaction in organic chemistry involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide. The reaction is named after Ivar Karl Ugi, who first reported this reaction in 1 .... References {{Reflist, 30em Rearrangement reactions Name reactions Carboximidates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Overman Rearrangement
The Overman rearrangement is a chemical reaction that can be described as a Claisen rearrangement of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate. The Overman rearrangement was discovered in 1974 by Larry Overman. The ,3sigmatropic rearrangement is diastereoselective and requires heating or the use of Hg(II) or Pd(II) salts as catalysts. The resulting allylamine structures can be transformed into many chemically and biologically important natural and un-natural amino acids (like (1- adamantyl)glycine). The Overman rearrangement may also be used for asymmetric synthesis.''Asymmetric Overman Rearrangement'' ''Organic Syntheses'', Vol. 82, p.134 (2005).Article) See also * Pinner reaction The Pinner reaction refers to the acid catalysed reaction of a nitrile with an alcohol to form an imino ester salt (alkyl imidate salt); this is sometimes referred to as a Pinner salt. The reaction is named after Adolf Pinner, who first describe ... ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2. Examples of amidines include: * DBU * diminazene * benzamidine * Pentamidine * Paranyline Preparation A common route to primary amidines is the Pinner reaction. Reaction of the nitrile with alcohol in the presence of acid gives an iminoether. Treatment of the resulting compound with ammonia then completes the conversion to the amidine. Instead of using a Bronsted acid, Lewis acids such as aluminium trichloride promote the direct amination of nitriles. They are also generated by amination of an imidoyl chloride. They are also prepared by the addition of organolithium reagents to diimines, followed by protonation or alkylation. Dimethylformamide acetal reacts with primary amines to give amidines: :Me2NC(H)(OMe)2 + RNH2 → Me2NC=NHR + 2 MeOH Propert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthoester
In organic chemistry, an ortho ester is a functional group containing three alkoxy groups attached to one carbon atom, i.e. with the general formula . Orthoesters may be considered as products of exhaustive alkylation of unstable orthocarboxylic acids and it is from these that the name 'ortho ester' is derived. An example is ethyl orthoacetate, , more correctly known as 1,1,1-triethoxyethane. Synthesis Ortho esters can be prepared by the Pinner reaction, in which nitriles react with alcohols in the presence of one equivalent of hydrogen chloride. The reaction proceeds by formation of imido ester hydrochloride: :RCN + R′OH + HCl → C(OR′)=NH2sup>+Cl− Upon standing in the presence of excess alcohol, this intermediate converts to the ortho ester: : C(OR′)=NH2sup>+Cl− + 2R′OH → RC(OR′)3 + NH4Cl The reaction requires anhydrous conditions. Although a less common method, ortho esters were first produced by reaction of 1,1,1-trichloroalkanes with sodium alkoxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Structure For ketimines and aldimines, respectively, the five core atoms (C2C=NX and C(H)C=NX, X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29-1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C-N distances in amines and nitriles are 1.47 and 1.16 Å, respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E- and Z-isomers of aldimines have been detected. Owing to steric effects, the E isomer is favored. Nomenclature and classification The term "imine" was coin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinner Übersicht Version 2
Pinner is a London suburb in the London borough of Harrow, Greater London, England, northwest of Charing Cross, close to the border with Hillingdon, historically in the county of Middlesex. The population was 31,130 in 2011. Originally a mediaeval hamlet, the St John Baptist church dates from the 14th century and other parts of the historic village include Tudor buildings. The newer High Street is mainly 18th-century buildings, while Bridge Street has a more urban character and many chain stores. History Pinner was originally a hamlet, first recorded in 1231 as ''Pinnora'', although the already archaic ''-ora'' (meaning 'hill') suggests its origins lie no later than circa 900. The name ''Pinn'' is shared with the River Pinn, which runs through the middle of Pinner. Another suggestion of the name is that it means 'hill-slope shaped like a pin'. The oldest part of the town lies around the fourteenth-century parish church of St. John the Baptist, at the junction of the prese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specific react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arthur William Chapman
Arthur is a common male given name of Brythonic origin. Its popularity derives from it being the name of the legendary hero King Arthur. The etymology is disputed. It may derive from the Celtic ''Artos'' meaning “Bear”. Another theory, more widely believed, is that the name is derived from the Roman clan '' Artorius'' who lived in Roman Britain for centuries. A common spelling variant used in many Slavic, Romance, and Germanic languages is Artur. In Spanish and Italian it is Arturo. Etymology The earliest datable attestation of the name Arthur is in the early 9th century Welsh-Latin text ''Historia Brittonum'', where it refers to a circa 5th to 6th-century Briton general who fought against the invading Saxons, and who later gave rise to the famous King Arthur of medieval legend and literature. A possible earlier mention of the same man is to be found in the epic Welsh poem ''Y Gododdin'' by Aneirin, which some scholars assign to the late 6th century, though this is still a m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Newman–Kwart Rearrangement
The Newman–Kwart rearrangement is a type of rearrangement reaction in which the aryl group of an ''O''-aryl thiocarbamate, ArOC(=S)NMe2, migrates from the oxygen atom to the sulfur atom, forming an ''S''-aryl thiocarbamate, ArSC(=O)NMe2. The reaction is named after its discoverers, Melvin Spencer Newman and Harold Kwart. The reaction is a manifestation of the double bond rule. : Mechanism The Newman–Kwart rearrangement is intramolecular; it proceeds ''via'' a four-membered cyclic transition state. : Use for preparation of thiophenols The Newman–Kwart rearrangement is an important prelude to the synthesis of thiophenols. A phenol (1) is deprotonated with a base followed by treatment with a thiocarbamoyl chloride (2) to form an ''O''-aryl thiocarbamate (3). Heating 3 to around 250 °C causes it undergo Newman–Kwart rearrangement to an ''S''-aryl thiocarbamate (4). Alkaline hydrolysis or similar cleavage yields a thiophenol (5). : See also * Smiles rearrangement Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |