|
Cannula Transfer
Cannula transfer or cannulation is a set of air-free techniques used with a Schlenk line, in transferring liquid or solution samples between reaction vessels via cannulae, avoiding atmospheric contamination. Syringes are not the same as cannulae, but cannula transfer techniques remain relevant when using them for this purpose. Two methods of cannula transfer are popular: vacuum, and pressure. Both utilize differences in pressures between two vessels to push the fluid through. Often, the main difficulty encountered is slow transfer due to the high viscosity of the fluid. Equipment Septa Septa (: septum) are rubber stoppers which seal flasks or bottles. They give an airtight seal, preventing the ingress of the atmosphere, but are able to be pierced by sharp needles or cannulae. Cannula Cannulae are hollow flexible tubes. Their bore is usually 16-22 gauge thick. They are commonly made of stainless steel or PTFE for their chemical resistance. Stainless steel cannulae are usually 2� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luer Taper
The Luer taper is a standardized system of small-scale fluid fittings used for making leak-free connections between a male-taper fitting and its mating female part on medical and laboratory instruments, including hypodermic syringe tips and needles or stopcocks and needles. Currently ISO 80369 governs the Luer standards and testing methods. History Named after the Lüer family who founded the medical instrument maker Maison Lüer in Paris, the two-piece syringe was invented by either the employed instrument maker Karl Schneider or Jeanne Lüer together with the glass blower Fournier. It was patented by Jeanne's husband Hermann Wülfing Lüer in 1894, with international patents taken out in the following years. It originated as a 6% taper fitting for glass bottle stoppers (so one side is 1.72 degrees from the centerline). Key features of Luer taper connectors are defined in the ISO 80369-7 standards. It is also defined in the DIN and EN standard 1707:1996 and 20594-1:1993. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company owned by the multinational chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company has two United States headquarters, in St. Louis and Burlington, MA and has operations in approximately 40 countries. In 2015, the multinational chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. It is headquartered in Burlington, Massachusetts, United States. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
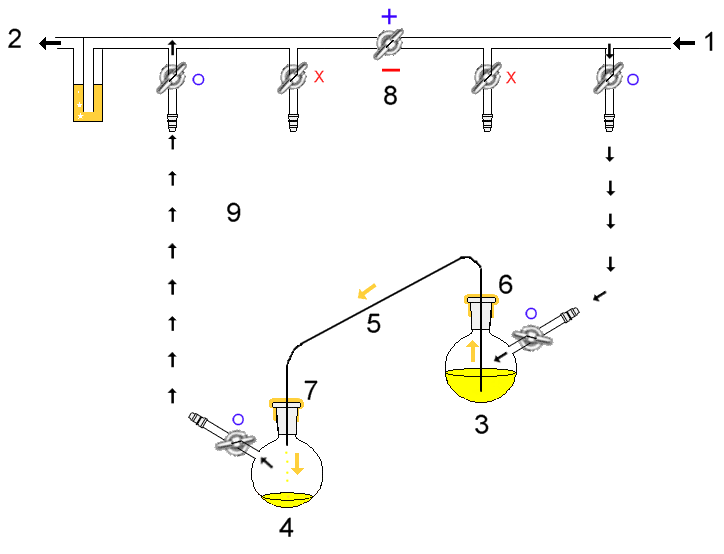
Air-free Technique
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen. The two most common types of air-free technique involve the use of a glovebox and a Schlenk line, although some rigorous applications use a high-vacuum line. In both methods, glassware (often Schlenk tubes) are pre-dried in ovens prior to use. They may be flame-dried to remove adsorbed water. Prior to coming into an inert atmosphere, vessels are further dried by ''purge-and-refill'' — the vessel is subjected to a vacuum to remove gases and water, and then refilled with inert gas. This cycle is usually ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syringe Filter
A syringe filter (sometimes called a wheel filter if it has a wheel-like shape) is a single-use filter cartridge. It is attached to the end of a syringe for use. Syringe filters may have Luer lock fittings, though not universally so. The use of a needle is optional; where desired it may be fitted to the end of the syringe filter. A syringe filter generally consists of a plastic housing with a membrane that serves as a filter. The fluid to be purified may be cleaned only by being pushed through the filter from syringe, it cannot be drawn into the syringe through the filter due to its one way process. Forms In scientific applications, the most common sizes available are 0.2 or 0.22 micrometre, μm and 0.45 μm pores. These sizes are sufficient for High-performance liquid chromatography, HPLC use. The smallest known sterile syringe microfilter have pore sizes of 0.02 μm. Membrane diameters of 10 mm, 13 mm, 25 mm are common as well. Some syringe filter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Google Books
Google Books (previously known as Google Book Search, Google Print, and by its code-name Project Ocean) is a service from Google that searches the full text of books and magazines that Google has scanned, converted to text using optical character recognition (OCR), and stored in its digital database.The basic Google book link is found at: https://books.google.com/ . The "advanced" interface allowing more specific searches is found at: https://books.google.com/advanced_book_search Books are provided either by publishers and authors through the Google Books Partner Program, or by Google's library partners through the Library Project. Additionally, Google has partnered with a number of magazine publishers to digitize their archives. The Publisher Program was first known as Google Print when it was introduced at the Frankfurt Book Fair in October 2004. The Google Books Library Project, which scans works in the collections of library partners and adds them to the digital inventory, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylaluminum
Trimethylaluminium or TMA is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula (abbreviated as , where Me stands for methyl), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium. Structure and bonding The structure and bonding in and diborane are analogous (R = alkyl). In , the Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively. The Al center is tetrahedral. The carbon atoms of the bridging methyl groups are each surrounded by five neighbors: three hydrogen atoms and two aluminium atoms. The methyl groups interchange readily intramolecularly. At higher temperatures, the dimer cracks into monomeric . Synthesis TMA is prepared via a two-step process that can be summarized as follows: : Applications Catalysis Starting with the invention of Ziegler-Natta catalysis, organoaluminium compounds have a prominent role in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-butyllithium
''tert''-Butyllithium is a chemical compound with the Chemical formula, formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base (chemistry), base, capable of deprotonation, deprotonating many carbon molecules, including benzene. ''tert''-Butyllithium is available commercially as solutions in hydrocarbons (such as pentane); it is not usually prepared in the laboratory. Preparation ''tert''-Butyllithium is produced commercially by treating tert-butyl chloride with lithium. Its synthesis was first reported by R. B. Woodward in 1941. Structure and bonding Like other organolithium compounds, ''tert''-butyllithium is a cluster compound. Whereas n-Butyllithium, ''n''-butyllithium exists both as a hexamer and a tetramer, ''tert''-butyllithium exists exclusively as a tetramer with a cubane-type cluster, cubane structure. Bonding in organolithium clusters involves three-center two-electron bond, sigma delocalization and significan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or (with a few exceptions) nitrogen. Fire classification fire extinguishers are designated for use in fires involving metals but not pyrophoric materials in general. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed. Uses The creation of sparks from metals is based on the pyrophoricity of small metal particles, and pyrophoric alloys are made for this purpose. Practical applications include the sparking mechanisms in lighters and various toys, using ferrocerium; starting fires without matches, using a firesteel; the flintlock m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syphon
A siphon (; also spelled syphon) is any of a wide variety of devices that involve the flow of liquids through tubes. In a narrower sense, the word refers particularly to a tube in an inverted "U" shape, which causes a liquid to flow upward, above the surface of a reservoir, with no pump, but powered by the fall of the liquid as it flows down the tube under the pull of gravity, then discharging at a level lower than the surface of the reservoir from which it came. There are two leading theories about how siphons cause liquid to flow uphill, against gravity, without being pumped, and powered only by gravity. The traditional theory for centuries was that gravity pulling the liquid down on the exit side of the siphon resulted in reduced pressure at the top of the siphon. Then atmospheric pressure was able to push the liquid from the upper reservoir, up into the reduced pressure at the top of the siphon, like in a barometer or drinking straw, and then over. However, it has been demon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Bubbler
A gas bubbler is a piece of laboratory glassware which consists of a glass bulb filled with a small amount of fluid—usually mineral oil, mineral or silicone oil, less commonly mercury. The inlet to the bulb is connected to a ground glass joint, while the outlet is vented to the air. Gas bubblers are used to exclude air from a reaction or a system. In the former case, the gas bubbler is fitted on the Condenser (laboratory), condenser of the reaction set-up. In the latter case, an oil bubbler is usually installed at the end of the inert gas manifold on a Schlenk line to prevent contamination by atmospheric oxygen and water. A gas bubbler acts as a one-way valve—gases (hot air, evolved gases, solvent vapors) from the inlet will bubble through the fluid before being vented to the atmosphere. If there were an underpressure in the reaction vessel (such as when heat is removed, and the gases within contract), some fluid is sucked into a sump to equalize the pressure, inst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |