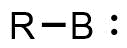|
Boron Monofluoride
Boron monofluoride or fluoroborylene is a chemical compound with the formula BF, one atom of boron and one of fluorine. It is an unstable gas, but it is a stable ligand on transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons. Structure The experimental B–F bond length is 1.26267 Å. Despite being isoelectronic to CO and N2, each of which is typically described as having a triple bond, computational studies generally agree that the true bond order is much lower than 3. One reported computed bond order for the molecule is 1.4, compared with 2.6 for CO and 3.0 for N2. BF is unusual in that the dipole moment is inverted, with fluorine having a positive charge even though it is the more e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monofluoride
Aluminium monofluoride, also known as fluoridoaluminium, is the chemical compound with the formula AlF. This elusive species is formed by the reaction between aluminium trifluoride and metallic aluminium at elevated temperatures but quickly reverts to the reactants when cooled. Clusters derived from related aluminium(I) halides can be stabilized using specialized ligands. This molecule has been detected in the interstellar medium The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It f ..., where molecules are so dilute that intermolecular collisions are unimportant. See also * Aluminium monobromide * Aluminium monochloride * Aluminium monoiodide References Aluminium(I) compounds Fluorides Metal halides Diatomic molecules {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borylene
A borylene is the boron analogue of a carbene. The general structure is R-B: with R an organic moiety (chemistry), moiety and B a boron atom with two unshared electrons. Borylenes are of academic interest in organoboron chemistry. A diradical, singlet ground state is predominant with boron having two vacant sp2 orbitals and one doubly occupied one. With just one additional substituent the boron is more electron deficient than the carbon atom in a carbene. For this reason stable borylenes are more uncommon than stable carbenes. Some borylenes such as boron monofluoride (BF) and boron monohydride (BH) the parent compound also known simply as borylene, have been detected in microwave spectroscopy and may exist in stars. Other borylenes exist as reactive intermediates and can only be inferred by chemical trapping. The first stable terminal borylene complex [(OC)5WBN(SiMe3)2] was reported by Holger Braunschweig et al. in 1998. In this compound a borylene is coordinated to a transition m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is Saturated and unsaturated compounds, unsaturated because its two carbon atoms are Chemical bond, bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. The triple bond in acetylene results in a high energy content that is released when acetylene is burned. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diboron Tetrafluoride
Diboron tetrafluoride is the inorganic compound with the formula (BF2)2, classed as a tetrahalodiborane. A colorless gas, the compound has a halflife of days at room temperature. It is the most stable of the diboron tetrahalides, and does not appreciably decompose under standard conditions. Structure and bonding Diboron tetrafluoride is a planar molecule with a B-B bond distance of 172 pm. Although it is electron-deficient, the unsaturated boron centers are stabilized by pi-bonding with the terminal fluoride ligands. The compound is isoelectronic with oxalate. Synthesis and reactions Diboron tetrafluoride can be formed by treating boron monofluoride with boron trifluoride at low temperatures, taking care not to form higher polymers. Alternatively, diboron tetrachloride can be fluorinated with antimony trifluoride Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swarts' reagent, it is one of two principal fluorides of antimony, the ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopic Constant
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and electronic structure of matter to be investigated at the atomic, molecular and macro scale, and over astronomical distances. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Current applications of spectroscopy include biomedical spectroscopy in the areas of tissue analysis and medical imaging. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pi Backbonding
In chemistry, pi backbonding or π backbonding is a π-bonding interaction between a filled (or half filled) orbital of a transition metal atom and a vacant orbital on an adjacent ion or molecule. In this type of interaction, electrons from the metal are used to bond to the ligand, which dissipates excess negative charge and stabilizes the metal. It is common in transition metals with low oxidation states that have ligands such as carbon monoxide, olefins, or phosphines. The ligands involved in π backbonding can be broken into three groups: carbonyls and nitrogen analogs, alkenes and alkynes, and phosphines. Compounds where π backbonding is prominent include Ni(CO)4, Zeise's salt, and molybdenum and iron dinitrogen complexes. Metal carbonyls, nitrosyls, and isocyanides The electrons are partially transferred from a d-orbital of the metal to anti-bonding molecular orbitals of CO (and its analogs). This electron-transfer strengthens the metal–C bond and weakens the C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and locatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Polarity
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole-dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alkaline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between the numbers of electron pairs in bonding and antibonding molecular orbitals. Bond order gives a rough indication of the stability of a bond. Isoelectronic species have the same bond order. Examples The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diatomic nitrogen N≡N, the bond order between the two nitrogen atoms is 3 (triple bond). In acetylene H–C≡C–H, the bond order between the two carbon atoms is also 3, and the C–H bond order is 1 ( single bond). In carbon monoxide, , the bond order between carbon and oxygen is 3. In thiazyl trifluoride , the bond order between sulfur and nitrogen is 3, and between sulfur and fluorine is 1. In diatomic oxygen O=O the bond order ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, single bonds or double bond, double bonds, with a bond order of three. The most common triple bond is in a nitrogen N2 molecule; the second most common is that between two carbon atoms, which can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as diphosphorus and carbon monoxide, are also triple bonded. In skeletal formula, skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms. Bonding Triple bonding can be explained in terms of orbital hybridization. In the case of acetylene, each carbon atom has two sp orbital, sp-orbitals and two p-orbitals. The two sp-orbitals are linear, with 180° bond angles, and occupy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |