|
Boranes
A borane is a compound with the formula although examples include multi-boron derivatives. A large family of boron hydride clusters is also known. In addition to some applications in organic chemistry, the boranes have attracted much attention as they exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes, are also a well developed class of compounds. pp 151-195 History The development of the chemistry of boranes led to innovations in synthetic methods as well as structure and bonding. First, new synthetic techniques were required to handle diborane and many of its derivatives, which are both pyrophoric and volatile. Alfred Stock invented the glass vacuum line for this purpose. The structure of diborane was correctly predicted in 1943 many years after its discovery. Interest in boranes increased during World War II due to the potential of uranium borohydride for enrichment of the uranium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carborane
Carboranes (or carbaboranes) are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these clusters are Polyhedron, polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes. In terms of scope, carboranes can have as few as 5 and as many as 14 atoms in the cage framework. The majority have two cage carbon atoms. The corresponding carbon, C-alkyl and boron, B-alkyl analogues are also known in a few cases. Structure and bonding Carboranes and boranes adopt 3-dimensional cage (Cluster chemistry, cluster) geometries in sharp contrast to typical organic compounds. Cages are compatible with sigma—delocalized bonding, whereas hydrocarbons are typically chains or rings. Like for other electron-delocalized polyhedral clusters, the electronic structure of these cluster compounds can be described by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
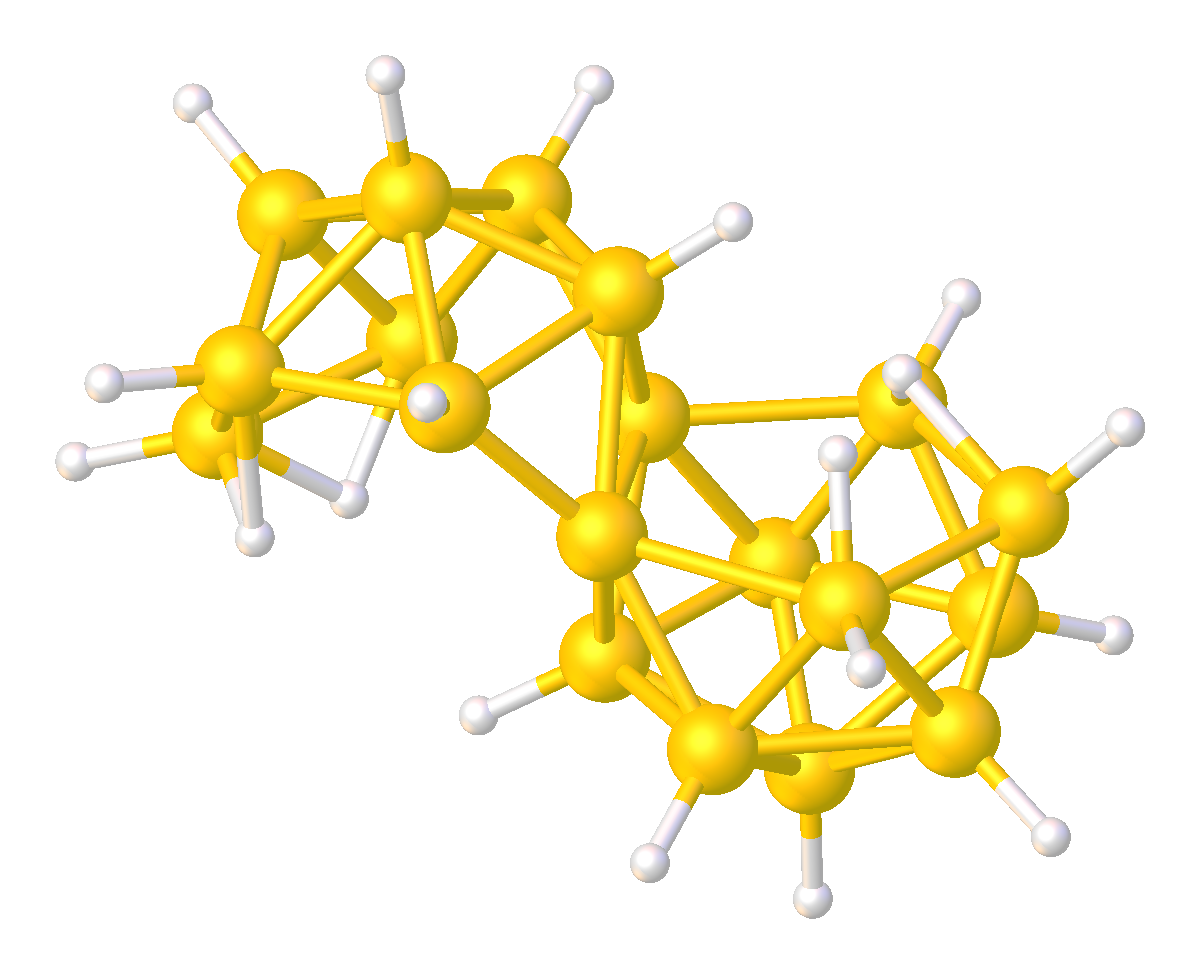
Boron Hydride Clusters
Boron hydride clusters are compounds with the formula or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are those with 5, 10, and 12 boron atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed. History The development of the borane hydride clusters resulted from pioneering work by Alfred Stock, invented the glass vacuum line for their study. The structures of the boron hydride clusters were determined beginning in 1948 with the characterization of decaborane. William Lipscomb was awarded the Nobel Prize in Chemistry in 1976 for this and many subsequent crystallographic investigations. These investigations revealed the prevalence of deltahedral structures, i.e., networks of triangular arrays of BH centers. The bonding of the clusters ushered in P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds. Hydroboration produces organoborane compounds that react with a variety of reagents to produce useful compounds, such as alcohols, amines, or alkyl halides. The most widely known reaction of the organoboranes is oxidation to produce alcohols from alkenes. The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry to Herbert C. Brown. Borane adducts Much of the original work on hydroboration employed diborane as a source of BH3. Usually however, borane dimethylsulfide complex BH3S(CH3)2 (BMS) is used instead. It can be obtained in highly concentrated forms. The adduct BH3(THF) is also commercially available as THF solutions. Its shelf life is less than BMS. In terms of synthetic results, dibor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Diisopinocampheylborane
Diisopinocampheylborane is an organoborane that is useful for asymmetric synthesis. This colourless solid is the precursor to a range of related reagents. The compound was reported in 1961 by Zweifel and Brown in a pioneering demonstration of asymmetric synthesis using boranes. The reagent is mainly used for the synthesis of chiral secondary alcohols. The reagent is often depicted as a monomer but like most hydroboranes, it is dimeric with B-H-B bridges. Preparation Diisopinocampheylborane was originally prepared by hydroboration of excess α-pinene with borane, but it is now more commonly generated from borane-methyl sulfide (BMS). The compound can be isolated as a solid, but because it is quite sensitive to water and air, it is often generated in situ and used as a solution. The synthesis is complicated by a number of factors, including the tendency of the compound to eliminate pinene. Diisopinocampheylborane is often represented as a monomer (including in this article), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Borane
Borane is an inorganic compound with the chemical formula . Because it tends to dimerize or form adducts, borane is very rarely observed. It normally dimerizes to diborane in the absence of other chemicals. It can be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. Structure and properties BH3 is a trigonal planar molecule with D3h symmetry. The experimentally determined B–H bond length is 119 pm. In the absence of other bases, it dimerizes to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction: :BX3 +BH4− → HBX3− + (BH3) (X=F, Cl, Br, I) :2 BH3 → B2H6 The standard enthalpy of dimerization of BH3 is estimated to be −170 kJ mol−1. The boron atom in BH3 has 6 valence electrons. Consequently, it is a strong Lewis acid and reacts with any Lewis base ('L' in equation below) to form an adduct: :BH3 + L � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Diborane
Diborane(6), commonly known as diborane, is the chemical compound with the formula . It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents. Structure and bonding The structure of diborane has D2h symmetry. Four hydrides are terminal, while two bridge between the boron centers. The lengths of the B–Hbridge bonds and the B–Hterminal bonds are 1.33 and 1.19 Å respectively. This difference in bond lengths reflects the difference in their strengths, the B–Hbridge bonds being relatively weaker. The weakness of the B–Hbridge compared to B–Hterminal bonds is indicated by their vibrational signatures in the infrared spectrum, being ≈2100 and 2500 cm−1 respectively. The model determined by molecular orbital theory describes the bonds between boron and the te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Triethylborane
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane. Preparation and structure Triethylborane is prepared by the reaction of trimethyl borate with triethylaluminium: :Et3Al + (MeO)3B → Et3B + (MeO)3Al The molecule is monomeric, unlike H3B and Et3Al, which tend to dimerize. It has a planar BC3 core. Applications Turbojet engines Triethylborane was used to ignite the JP-7 fuel in the Pratt & Whitney J58 turbojet/ ramjet engines powering the Lockheed SR-71 Blackbird and its predecessor, the A-12 OXCART. Triethylborane is suitable because it ignites readily upon exposure to oxygen. It was chosen as an ignition method for reliability reasons, and in the case of the Blackbird, because JP-7 fuel has very low volatility and is difficult to ignite. Conventional ignition plugs posed a high r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Triethylboron
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane. Preparation and structure Triethylborane is prepared by the reaction of trimethyl borate with triethylaluminium: :Et3Al + (MeO)3B → Et3B + (MeO)3Al The molecule is monomeric, unlike H3B and Et3Al, which tend to dimerize. It has a planar BC3 core. Applications Turbojet engines Triethylborane was used to ignite the JP-7 fuel in the Pratt & Whitney J58 turbojet/ramjet engines powering the Lockheed SR-71 Blackbird and its predecessor, the A-12 OXCART. Triethylborane is suitable because it ignites readily upon exposure to oxygen. It was chosen as an ignition method for reliability reasons, and in the case of the Blackbird, because JP-7 fuel has very low volatility and is difficult to ignite. Conventional ignition plugs posed a high risk of m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Triphenylboron
Triphenylborane is an chemical compound with the chemical formula , often abbreviated to , where Ph is the phenyl group. It is a white crystalline solid and is both air and moisture sensitive, slowly forming benzene and triphenylboroxine. It is soluble in aromatic solvents. Structure and properties The core of the compound, , has a trigonal planar structure. The phenyl groups are rotated at about a 30° angle from the core plane. Even though triphenylborane and tris(pentafluorophenyl)borane are structurally similar, their Lewis acidity is not. is a weak Lewis acid while is a strong Lewis acid due to the electronegativity of the fluorine atoms. Other boron Lewis acids include and . Synthesis Triphenylborane was first synthesized in 1922. It is typically made with boron trifluoride diethyl etherate and the Grignard reagent, phenylmagnesium bromide. : Triphenylborane can also be synthesized on a smaller scale by the thermal decomposition of trimethylammonium tetraphenylbora ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Trimethylboron
Trimethylborane (TMB) is a toxic, pyrophoric gas with the formula B(CH3)3 (which can also be written as Me3B, with Me representing methyl). Properties As a liquid it is colourless. The strongest line in the infrared spectrum is at 1330 cm−1 followed by lines at 3010 cm−1 and 1185 cm−1. Its melting point is −161.5 °C, and its boiling point is −20.2 °C. Vapour pressure is given by , where ''T'' is temperature in kelvins. Molecular weight is 55.914. The heat of vapourisation is 25.6 kJ/mol. Preparation Trimethylborane was first described in 1862 by Edward Frankland, who also mentioned its adduct with ammonia. Due to its dangerous nature the compound was no longer studied until 1921, when Alfred Stock and Friedrich Zeidler took advantage of the reaction between boron trichloride gas and dimethylzinc. Although the substance can be prepared using Grignard reagents the output is contaminated by unwanted products from the solvent. Trimethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
9-BBN
9-Borabicyclo .3.1onane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates. 9-BBN is also known by its nickname 'banana borane'. This is because rather than drawing out the full structure, chemists often simply draw a banana shape with the bridging boron. Preparation 9-BBN is prepared by the reaction of 1,5-cyclooctadiene and borane usually in ethereal solvents, for example: The compound is commercially available as a solution in tetrahydrofuran and as a solid. 9-BBN is especially useful in Suzuki reactions. Its highly regioselective addition on alkenes allows the preparation of terminal alcohols by subsequent oxidative cleavage with H2O2 in aqueous KOH. The steric demand of 9-BBN greatly suppresses the formation of the 2-substituted isomer compared to the use of borane. See also * Organoboron chemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Thexylborane
Thexylborane is a borane with the formula e2CHCMe2BH2sub>2 (Me = methyl). The name derives from "''t''-hexylborane" (although the group is not the standard ''tert''-hexyl group), and the formula is often abbreviated ThxBH2. A colorless liquid, it is a monoalkylborane. It is produced by the hydroboration of tetramethylethylene: :B2H6 + 2 Me2C=CMe2 → e2CHCMe2BH2sub>2 Reactions Thexylborane is generated ''in situ is a Latin phrase meaning 'in place' or 'on site', derived from ' ('in') and ' ( ablative of ''situs'', ). The term typically refers to the examination or occurrence of a process within its original context, without relocation. The term is use ...''. In solution, it isomerizes over the course several days to the 2,3-dimethyl-1-butyl derivative, shown as the monomer here: :Me2CHCMe2BH2 → Me2CHCH(Me)CH2BH2 Thexylborane allows the synthesis of ketones by coupling a pair of alkenes with carbon monoxide, which serves as a carbonyl linchpin: :Me2CHCMe2BH2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |