|
Bioorganometallic Chemistry
Bioorganometallic chemistry is the study of biologically active molecules that contain carbon directly bonded to metals or metalloids. The importance of main-group and transition-metal centers has long been recognized as important to the function of enzymes and other biomolecules. However, only a small subset of naturally-occurring metal complexes and synthetically prepared pharmaceuticals are ''organometallic''; that is, they feature a direct covalent bond between the metal(loid) and a carbon atom. The first, and for a long time, the only examples of naturally occurring bioorganometallic compounds were the cobalamin cofactors (vitamin B12) in its various forms. In the 21st century, as a result of the discovery of new systems containing carbon–metal bonds in biology, bioorganometallic chemistry is rapidly emerging as a distinct subdiscipline of bioinorganic chemistry that straddles organometallic chemistry and biochemistry. Naturally occurring bioorganometallics include enzyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. One of eight B vitamins, it serves as a vital cofactor (biochemistry), cofactor in DNA synthesis and both fatty acid metabolism, fatty acid and amino acid metabolism. It plays an essential role in the nervous system by supporting myelinogenesis, myelin synthesis and is critical for the maturation of red blood cells in the bone marrow. While animals require B12, plants do not, relying instead on alternative enzymatic pathways. Vitamin B12 is the most chemically complex of all vitamins, and is synthesized exclusively by certain archaea and bacteria. Natural food sources include meat, shellfish, liver, fish, poultry, Egg as food, eggs, and dairy products. It is also added to many breakfast cereals through food fortification and is available in dietary supplement and pharmaceutical forms. Supplements are commonly taken orally but may be administered via intramuscular injection to treat defic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
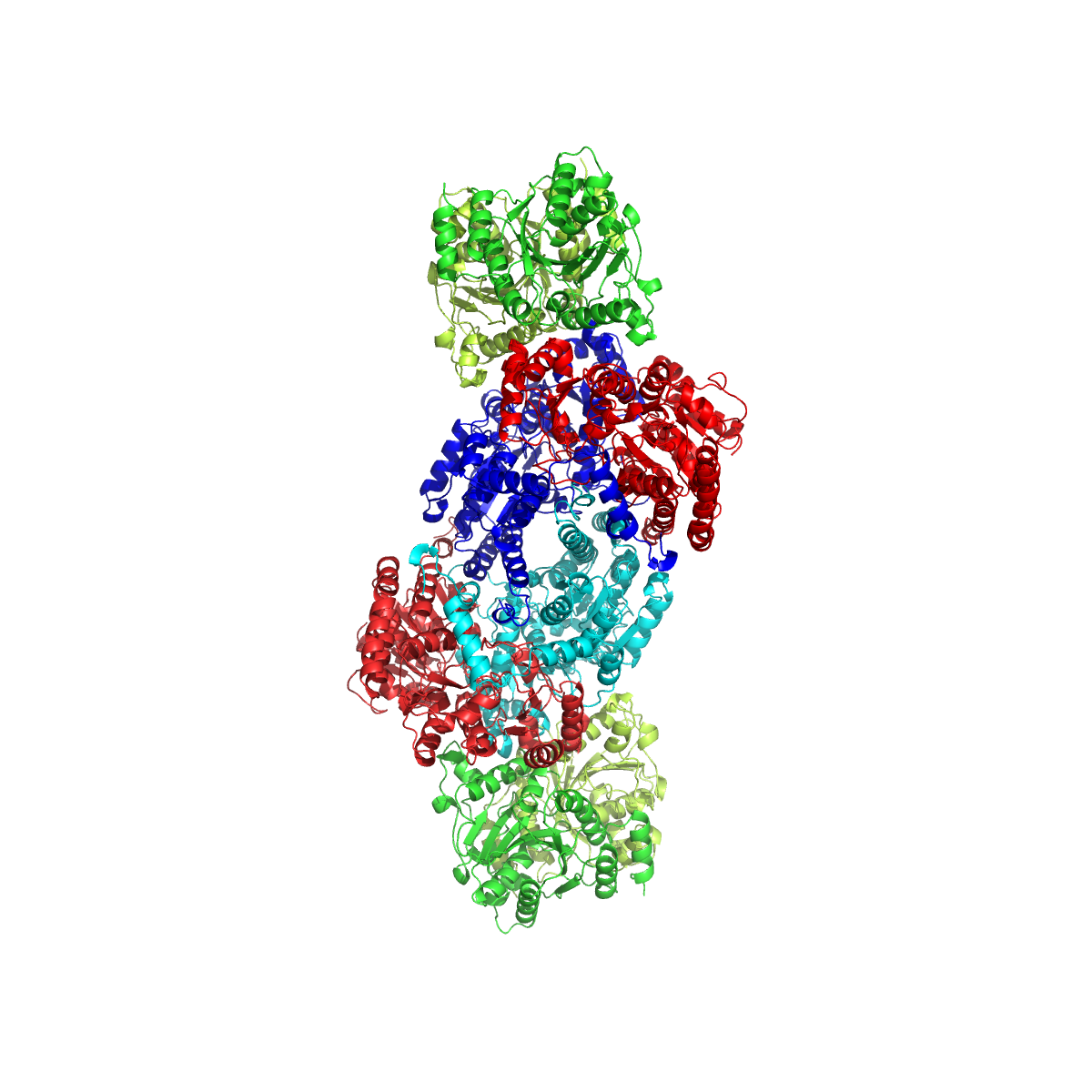
Lactate Racemase
The lactate racemase enzyme (Lar) () interconverts the D- and L-enantiomers of lactic acid. It is classified under the isomerase, racemase, epimerase, and enzyme acting on hydroxyl acids and derivatives classes of enzymes. It is found in certain halophilic archaea, such as ''Haloarcula marismortui'', and in a few species of bacteria, such as several ''Lactobacillus'' species (which produce D- and L- lactate) including ''Lactobacillus sakei'', ''Lactobacillus curvatus'', and ''Lactobacillus plantarum'', as well as in non-lactic acid bacteria such as ''Clostridium beijerinckii''. The gene encoding lactate racemase in ''L. plantarum'' was identified as ''larA'' and shown to be associated with a widespread maturation system involving ''larB'', ''larC1'', ''larC2'', and ''larE''. The optimal pH for its activity is 5.8-6.2 in ''L. sakei''. Structure and properties The molecular weight of lactate racemase differs in the various organisms in which it has been found, ranging from 25,0 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece. Interstitial / Metallic carbides The carbides of the group 4, 5 and 6 transition metals (with the exception of chromium) are often described as interstitial compounds. These carbides have metallic properties and are refractory. Some exhibit a range of stoichiometries, being a non-stoichiometric mixture of various carbides arising due to crystal defects. Some of them, including titanium carbide and tungsten carbide, are important industrially and are used to coat metals in cutting tools. The long-held view is that the carbon atoms fit into octahedral interstices in a close-packed metal lattice when the metal atom radius is greater than approximately 135 pm: *When the metal atoms are cubic close-packed, (ccp), then filling all of the octahedral interstices with carbon achieves 1:1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules (nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase. Classification and structure Although the equilibrium formation of ammonia from molecular hydrogen and nitrogen has an overall negative enthalpy of reaction ( \Delta H^ = -45.2 \ \mathrm \, \mathrm \; \mathrm ), the activation energy is very high ( E_\mathrm = 230-420 \ \mathrm \, \mathrm ). Nitrogenase acts as a catalyst, reducing this energy barrie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FeMoco
FeMoco ( cofactor) or M-cluster is the primary cofactor of nitrogenase. Nitrogenase is the enzyme that catalyzes the conversion of atmospheric nitrogen molecules N2 into ammonia (NH3) through the process known as nitrogen fixation. Because it contains iron and molybdenum, the cofactor is called FeMoco. Its stoichiometry is Fe7MoS9C. Structure The FeMo cofactor is a cluster with composition Fe7MoS9C. This cluster can be viewed as two subunits composed of one Fe4S3 ( iron(III) sulfide) cluster and one MoFe3S3 cluster. The two clusters are linked by three sulfide ligands and a bridging carbon atom. The unique iron (Fe) is anchored to the protein by a cysteine. It is also bound to three sulfides, resulting in tetrahedral molecular geometry. The additional six Fe centers in the cluster are each bonded to three sulfides. These six internal Fe centers define a trigonal prismatic arrangement around a central carbide center. The molybdenum is attached to three sulfides and is anchored ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor F430
F430 is the cofactor (sometimes called the coenzyme) of the enzyme methyl coenzyme M reductase (MCR). MCR catalyzes the reaction that releases methane in the final step of methanogenesis: : + HS–CoB → + CoB–S–S–CoM It is found only in methanogenic Archaea and anaerobic methanotrophic Archaea. It occurs in relatively high concentrations in archaea that are involved in reverse methanogenesis: these can contain up to 7% by weight of the nickel protein. Structure The trivial name cofactor F430 was assigned in 1978 based on the properties of a yellow sample extracted from '' Methanobacterium thermoautotrophicum'', which had a spectroscopic maximum at 430 nm. It was identified as the MCR cofactor in 1982 and the complete structure was deduced by X-ray crystallography and NMR spectroscopy. Coenzyme F430 features a reduced porphyrin in a macrocyclic ring system called a corphin. In addition, it possesses two additional rings in comparison to the standard tetrapyrrole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classifie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanogenesis
Methanogenesis or biomethanation is the formation of methane coupled to energy conservation by microbes known as methanogens. It is the fourth and final stage of anaerobic digestion. Organisms capable of producing methane for energy conservation have been identified only from the domain Archaea, a group phylogenetically distinct from both eukaryotes and bacteria, although many live in close association with anaerobic bacteria. The production of methane is an important and widespread form of microbial metabolism. In anoxic environments, it is the final step in the decomposition of biomass. Methanogenesis is responsible for significant amounts of natural gas accumulations, the remainder being thermogenic. Biochemistry Methanogenesis in microbes is a form of anaerobic respiration. Methanogens do not use oxygen to respire; in fact, oxygen inhibits the growth of methanogens. The terminal electron acceptor in methanogenesis is not oxygen, but carbon. The two best described pathways ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dalton Transactions
''Dalton Transactions'' is a weekly peer-reviewed scientific journal covering original (primary) research and review articles on all aspects of the chemistry of inorganic, bioinorganic, and organometallic compounds. It is published by the Royal Society of Chemistry and the editor-in-chief is Russell Morris (University of St Andrews). The journal was named after the English chemist, John Dalton, best known for his work on modern atomic theory. The journal was named a "rising star" in 2006. Publication history The journal was established as the ''Journal of the Chemical Society A: Inorganic, Physical, Theoretical'' in 1966. In 1972, the journal was divided into three separate journals: ''Journal of the Chemical Society, Dalton Transactions'' (covering inorganic and organometallic chemistry), '' Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases'', and '' Journal of the Chemical Society, Faraday Transactions 2: Molecular and Chemical Ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but both D and L-cysteine are found in nature. LCysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems. Cysteine is named after its discovery in urine, which comes from the urinary bladder or cyst, from Ancient Greek, Greek κύστις ''kýstis'', "bladder". The thiol is susceptible to oxidation to give the disulfide bond, disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. The deprotonated form can generally be described by the symbol Cym as well. When used as a food additive, cysteine has the E number E920. Cysteine is Genetic code, encoded by the codo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |