|
Binding Affinity
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the def ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
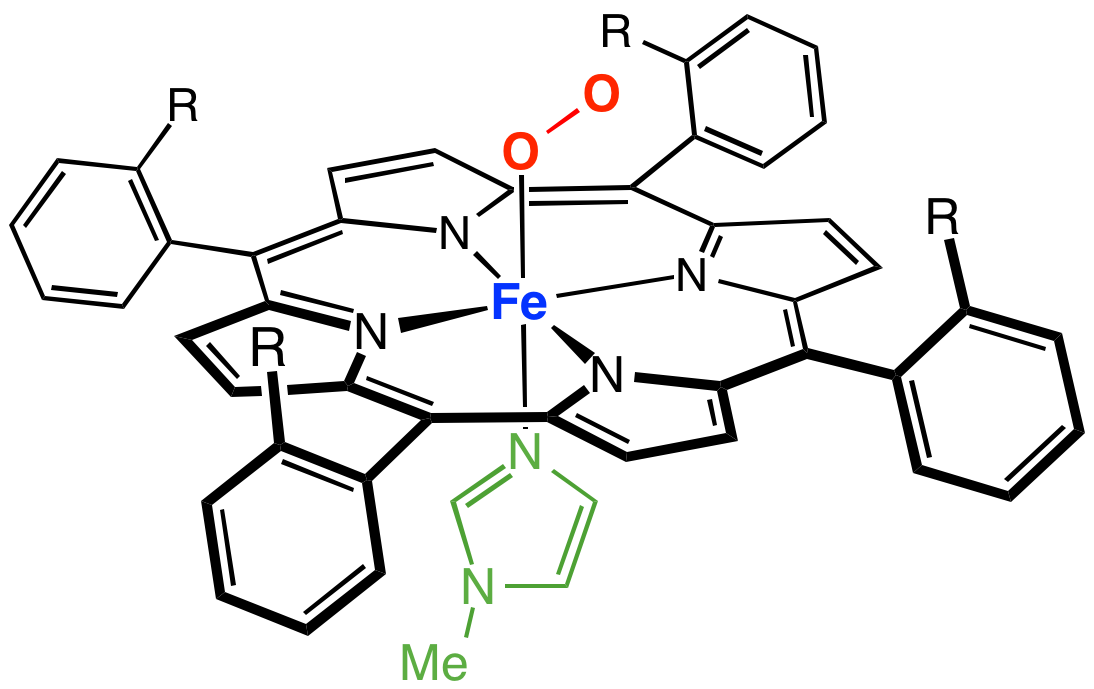
Myoglobin And Heme
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobin has a higher affinity for oxygen and does not have cooperative binding with oxygen like hemoglobin does. Myoglobin consists of non-polar amino acids at the core of the globulin, where the heme group is non-covalently bounded with the surrounding polypeptide of myoglobin. In humans, myoglobin is found in the bloodstream only after muscle injury. (Google books link is the 2008 edition) High concentrations of myoglobin in muscle cells allow organisms to hold their breath for a longer period of time. Diving mammals such as whales and seals have muscles with particularly high abundance of myoglobin. Myoglobin is found in Type I muscle, Type II A, and Type II B; although many older texts describe myoglobin as not found in smooth muscle, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Force
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the van der ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
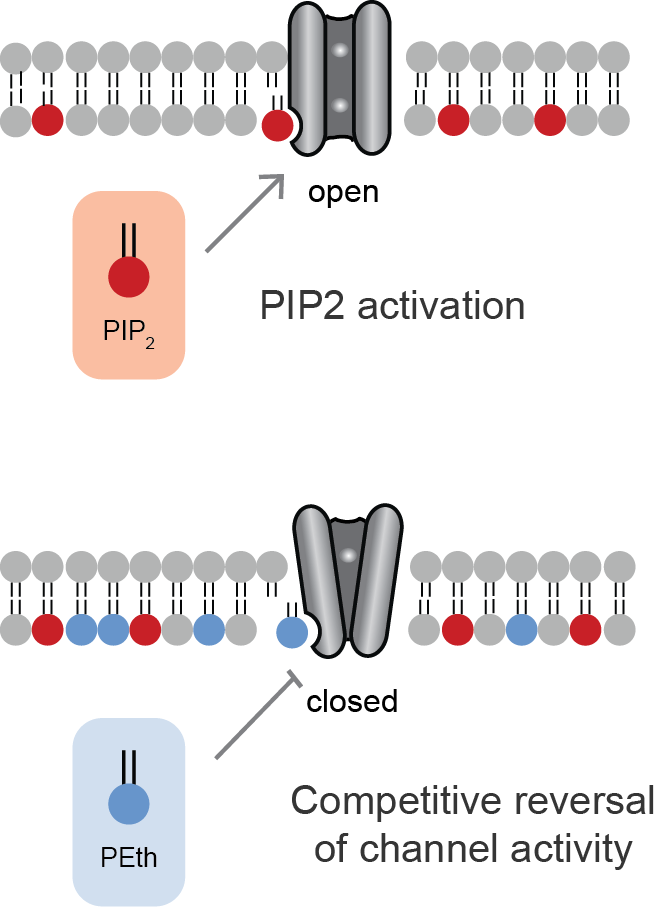
Lipid-gated Ion Channels
Lipid-gated ion channels are a class of ion channels whose conductance of ions through the membrane depends directly on lipids. Classically the lipids are membrane resident anionic signaling lipids that bind to the transmembrane domain on the inner leaflet of the plasma membrane with properties of a classic ligand. Other classes of lipid-gated channels include the mechanosensitive ion channels that respond to lipid tension, thickness, and hydrophobic mismatch. A lipid ligand differs from a lipid Cofactor (biochemistry), cofactor in that a Ligand (biochemistry), ligand derives its function by dissociating from the channel while a cofactor typically derives its function by remaining bound. PIP2-gated channels Phosphatidylinositol 4,5-bisphosphate (PIP2) was the first and remains the best studied lipid to gate ion channels. PIP2 is a cell membrane lipid, and its role in gating ion channels represents a novel role for the molecule. Kir channels: PIP2 binds to and directly activate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activator (proteomics)
Enzyme activators are molecules that bind to enzymes and increase their activity. They are the opposite of enzyme inhibitors. These molecules are often involved in the allosteric regulation of enzymes in the control of metabolism. In some cases, when a substrate binds to one catalytic subunit of an enzyme, this can trigger an increase in the substrate affinity as well as catalytic activity in the enzyme's other subunits, and thus the substrate acts as an activator. Examples Phosphofructokinase 1 An example of an enzyme activator working in this way is fructose 2,6-bisphosphate, which activates phosphofructokinase 1 and increases the rate of glycolysis in response to the hormone glucagon. Hexokinase-I Hexokinase-I (HK-I) is an enzyme activator because it draws glucose into the glycolysis pathway. Its function is to phosphorylate glucose releasing glucose-6-phosphate (G6P) as the product. HK-I not only signals the activation of glucose into glycolysis but also maintai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibitor
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its Enzyme activity, activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which Substrate (biochemistry), substrate molecules are converted into Product (chemistry), products. An enzyme Enzyme catalysis, facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the Rate-determining step, most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind Reversible reaction, reversibly or irreversibly. Irreversible inhibitors form a Covalent bond, chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substrate (biochemistry)
In chemistry, the term substrate is highly context-dependent. Broadly speaking, it can refer either to a chemical species being observed in a chemical reaction, or to a surface on which other chemical reactions or microscopy are performed. In the former sense, a reagent is added to the ''substrate'' to generate a product through a chemical reaction. The term is used in a similar sense in synthetic and organic chemistry, where the substrate is the chemical of interest that is being modified. In biochemistry, an enzyme substrate is the material upon which an enzyme acts. When referring to Le Chatelier's principle, the substrate is the reagent whose concentration is changed. ;Spontaneous reaction : :*Where S is substrate and P is product. ;Catalysed reaction : :*Where S is substrate, P is product and C is catalyst. In the latter sense, it may refer to a surface on which other chemical reactions are performed or play a supporting role in a variety of spectroscopic and mic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and Signal_transduction, transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and produce physiological responses, such as a change in the electrophysiology, electrical activity of a cell. For example, GABA, an inhibitory neurotransmitter, inhibits electrical activity of neurons by binding to GABAA receptor, GABA receptors. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand (biochemistry), ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Cell surface receptors, also known as transmembrane receptors, include ligand-gated ion channels, G prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the other tissues of the body, where it releases the oxygen to enable aerobic respiration which powers an animal's metabolism. A healthy human has 12to 20grams of hemoglobin in every 100mL of blood. Hemoglobin is a metalloprotein, a chromoprotein, and a globulin. In mammals, hemoglobin makes up about 96% of a red blood cell's dry matter, dry weight (excluding water), and around 35% of the total weight (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL of O2 per gram, which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood plasma alone. The mammalian hemoglobin molecule can bind and transport up to four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against nonmetallic materials which do not. Metals are typically ductile (can be drawn into a wire) and malleable (can be shaped via hammering or pressing). A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polythiazyl, polymeric sulfur nitride. The general science of metals is called metallurgy, a subtopic of materials science; aspects of the electronic and thermal properties are also within the scope of condensed matter physics and solid-state chemistry, it is a multidisciplinary topic. In colloquial use materials such as steel alloys are referred to as metals, while others such as polymers, wood or ceramics are nonmetallic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry
Inorganic chemistry deals with chemical synthesis, synthesis and behavior of inorganic compound, inorganic and organometallic chemistry, organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, pharmaceutical drug, medications, fuels, and agriculture. Occurrence Many inorganic compounds are found in nature as minerals. Soil may contain iron sulfide as pyrite or calcium sulfate as gypsum. Inorganic compounds are also found multitasking as biomolecules: as electrolytes (sodium chloride), in energy storage (Adenosine triphosphate, ATP) or in construction (the polyphosphate backbone in DNA). Bonding Inorganic compounds exhibit a range ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalorganic
Metal-organic compounds (jargon: metalorganics, metallo-organics) are a class of chemical compounds that contain metals and organic ligands, but lacking direct metal-carbon bonds. Metal β-diketonates, metal alkoxides, metal dialkylamides, transition metal carboxylate complexes, metal acetylacetonates, and metal phosphine complexes are representative members of this class. Some of metal-organic compounds confer solubility in organic solvents or volatility. Compounds with these properties find applications in materials science for metal organic vapor deposition (MOCVD) or sol-gel processing. Precise definitions of metal-organic compound may vary, however the term may describe: *Organometallic chemistry * Metal coordination complex A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' .. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |