|
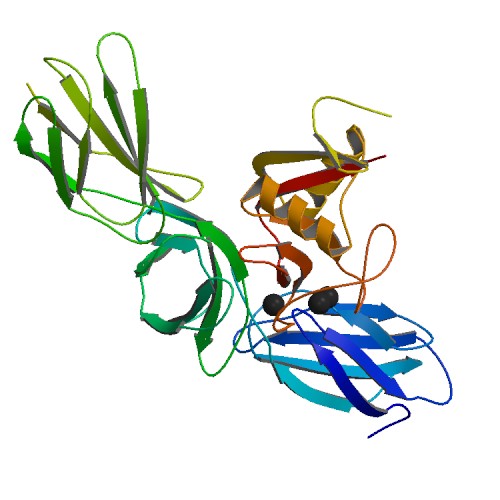
Biglycan
Biglycan is a small leucine-rich repeat proteoglycan (SLRP) which is found in a variety of extracellular matrix tissues, including bone, cartilage and tendon. In humans, biglycan is encoded by the ''BGN'' gene which is located on the X chromosome. The name "biglycan" was proposed in an article by Fisher, Termine and Young in an article in the Journal of Biological Chemistry in 1989 because the proteoglycan contained two GAG chains; formerly it was known as proteoglycan-I (PG-I). Structure Biglycan consists of a protein core containing leucine-rich repeat regions and two glycosaminoglycan (GAG) chains consisting of either chondroitin sulfate (CS) or dermatan sulfate (DS), with DS being more abundant in most connective tissues. The CS/DS chains are attached at amino acids 5 and 10 in human biglycan. The composition of the GAG chains has been reported as varying according to tissue of origin. Non-glycanated forms of biglycan (no GAG chains) increase with age in human articular c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decorin
Decorin is a protein that in humans is encoded by the ''DCN'' gene. Decorin is a proteoglycan that is on average 90 - 140 kilodaltons (kDa) in molecular weight. It belongs to the small leucine-rich proteoglycan (SLRP) family and consists of a protein core containing leucine repeats with a glycosaminoglycan (GAG) chain consisting of either chondroitin sulfate (CS) or dermatan sulfate (DS). Decorin is a small cellular or pericellular matrix proteoglycan and is closely related in structure to biglycan protein. Decorin and biglycan are thought to be the result of a gene duplication. This protein is a component of connective tissue, binds to type I collagen fibrils, and plays a role in matrix assembly. Naming Decorin's name is a derivative of both the fact that it "decorates" collagen type I, and that it interacts with the "d" and "e" bands of fibrils of this collagen. Function Decorin appears to influence fibrillogenesis, and also interacts with fibronectin, thrombospo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycans
Proteoglycans are proteins that are heavily glycosylation, glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalent bond, covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate-GlcA-Galactose, Gal-Gal-Xylose, Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser-Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate- GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser- Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SGCA
Alpha-sarcoglycan is a protein that in humans is encoded by the ''SGCA'' gene. Function The dystrophin-glycoprotein complex (DGC) comprises a group of proteins that are critical to the stability of muscle fiber membranes and to the linking of the actin cytoskeleton to the extracellular matrix. Components of the DGC include dystrophin (MIM 300377), which is deficient in Duchenne muscular dystrophy (DMD; MIM 310200); syntrophins (e.g., MIM 600026); dystroglycans (MIM 128239); and sarcoglycans, such as adhalin, a 50-kD transmembrane protein (Roberds et al., 1993). upplied by OMIM Clinical significance Mutations in the SGCA gene are known to cause Limb-girdle muscular dystrophy, autosomal recessive 3 (LGMDR3). This condition causes progressive muscle wasting from early childhood leading to loss of independent mobility as a teenager. Interactions SGCA has been shown to interact with Biglycan. References Further reading * * * * * * * * * * * * * * * * * External links *LOVD T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TGF-beta 1
Transforming growth factor beta 1 or TGF-β1 is a polypeptide member of the transforming growth factor beta superfamily of cytokines. It is a secreted protein that performs many cellular functions, including the control of cell growth, cell proliferation, cell differentiation, and apoptosis. In humans, TGF-β1 is encoded by the gene. Function TGF-β is a multifunctional set of peptides that controls proliferation, differentiation, and other functions in many cell types. TGF-β acts synergistically with transforming growth factor-alpha (TGF-α) in inducing transformation. It also acts as a negative autocrine growth factor. Dysregulation of TGF-β activation and signaling may result in apoptosis. Many cells synthesize TGF-β and almost all of them have specific receptors for this peptide. TGF-β1, TGF-β2, and TGF-β3 all function through the same receptor signaling systems. TGF-β1 was first identified in human platelets as a protein with a molecular mass of 25 kilodal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine-rich Repeat
A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe tertiary structure, fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These Protein tandem repeats, tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta sheet, beta strand-turn (biochemistry), turn-alpha helix structure, and the assembled structural domain, domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues. Leucine-rich repeats are frequently involved in the formation of protein–pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Growth Factors
A growth factor is a naturally occurring substance capable of stimulating cell proliferation, wound healing, and occasionally cellular differentiation. Usually it is a secreted protein or a steroid hormone. Growth factors are important for regulating a variety of cellular processes. Growth factors typically act as cell signaling, signaling molecules between cells. Examples are cytokines and hormones that bind to specific receptor (biochemistry), receptors on the surface of their target cell (biology), cells. They often promote cell differentiation and maturation, which varies between growth factors. For example, epidermal growth factor (EGF) enhances osteogenic differentiation (osteogenesis or bone formation), while fibroblast growth factors and vascular endothelial growth factors stimulate blood vessel differentiation (angiogenesis). Comparison to cytokines ''Growth factor'' is sometimes used interchangeably among scientists with the term ''cytokine.'' Historically, cytok ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atherosclerosis
Atherosclerosis is a pattern of the disease arteriosclerosis, characterized by development of abnormalities called lesions in walls of arteries. This is a chronic inflammatory disease involving many different cell types and is driven by elevated blood levels of cholesterol. These lesions may lead to narrowing of the arterial walls due to buildup of atheromatous plaques. At the onset, there are usually no symptoms, but if they develop, symptoms generally begin around middle age. In severe cases, it can result in coronary artery disease, stroke, peripheral artery disease, or kidney disorders, depending on which body part(s) the affected arteries are located in the body. The exact cause of atherosclerosis is unknown and is proposed to be multifactorial. Risk factors include dyslipidemia, abnormal cholesterol levels, elevated levels of inflammatory biomarkers, high blood pressure, diabetes, smoking (both active and passive smoking), obesity, genetic factors, family history, lifes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipoprotein
A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid (also known as fat) molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, surrounded by a phospholipid outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions oriented inward toward the lipid center. A special kind of protein, called apolipoprotein, is embedded in the outer shell, both stabilising the complex and giving it a functional identity that determines its role. Plasma lipoprotein particles are commonly divided into five main classes, based on size, lipid composition, and apolipoprotein content. They are, in increasing size order: HDL, LDL, IDL, VLDL and chylomicrons. Subgroups of these plasma particles are primary drivers or modulators of atherosclerosis. Many enzymes, transporters, structural proteins, antigens, adhesins, and toxins are some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen Type I
Type I collagen is the most abundant collagen of the human body, consisting of around 90% of the body's total collagen in vertebrates. Due to this, it is also the most abundant protein type found in all vertebrates. Type I forms large, eosinophilic fibers known as collagen fibers, which make up most of the rope-like dense connective tissue in the body. Collagen I itself is created by the combination of both a proalpha1 and a proalpha2 chain created by the COL1alpha1 and COL1alpha2 genes respectively. The Col I gene itself takes up a triple-helical conformation due to its Glycine-X-Y structure, x and y being any type of amino acid. Collagen can also be found in two different isoforms, either as a homotrimer or a heterotrimer, both of which can be found during different periods of development. Heterotrimers, in particular, play an important role in wound healing, and are the dominant isoform found in the body. Type I collagen can be found in a myriad of different places in the b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |