|
Accelerator Mass Spectrometry
Accelerator mass spectrometry (AMS) is a form of mass spectrometry that accelerates ions to extraordinarily high kinetic energies before mass analysis. The special strength of AMS among the mass spectrometric methods is its power to separate a rare isotope from an abundant neighboring mass ("abundance sensitivity", e.g. 14C from 12C). The method suppresses molecular isobars completely and in many cases can separate atomic isobars (e.g. 14N from 14C) also. This makes possible the detection of naturally occurring, long-lived radio-isotopes such as 10Be, 36Cl, 26Al and 14C. Their typical isotopic abundance ranges from 10−12 to 10−18. AMS can outperform the competing technique of decay counting for all isotopes where the half-life is long enough. Other advantages of AMS include its short measuring time as well as its ability to detect atoms in extremely small samples. The method Generally, negative ions are created (atoms are ionized) in an ion source. In for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionized, for example by bombarding it with a beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragments) are then separated acco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Abundance
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass (a weighted average, weighted by mole-fraction abundance figures) of these isotopes is the atomic weight listed for the element in the periodic table. The abundance of an isotope varies from planet to planet, and even from place to place on the Earth, but remains relatively constant in time (on a short-term scale). As an example, uranium has three naturally occurring isotopes: 238U, 235U and 234U. Their respective natural mole-fraction abundances are 99.2739–99.2752%, 0.7198–0.7202%, and 0.0050–0.0059%. For example, if 100,000 uranium atoms were analyzed, one would expect to find approximately 99,274 238U atoms, approximately 720 235U atoms, and very few (most likely 5 or 6) 234U atoms. This is because 238U is much more stable than 235U or 234U, as the half-life of each isotope reveals: 4.468 × 109 years for 238U co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
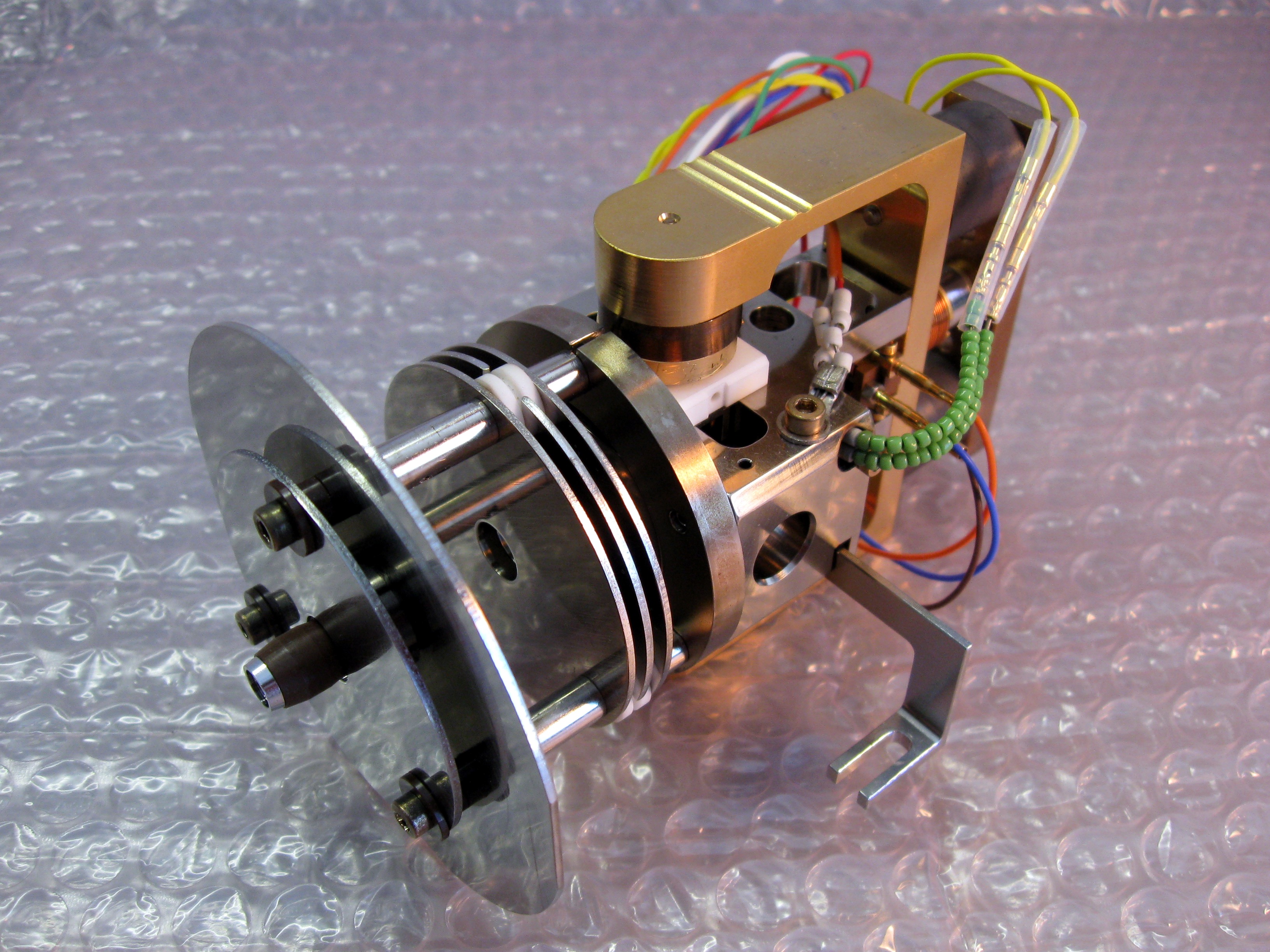
12929 2008 Article 54 Fig1 HTML
1 (one, unit, unity) is a number representing a single or the only entity. 1 is also a numerical digit and represents a single unit of counting or measurement. For example, a line segment of ''unit length'' is a line segment of length 1. In conventions of sign where zero is considered neither positive nor negative, 1 is the first and smallest positive integer. It is also sometimes considered the first of the infinite sequence of natural numbers, followed by 2, although by other definitions 1 is the second natural number, following 0. The fundamental mathematical property of 1 is to be a multiplicative identity, meaning that any number multiplied by 1 equals the same number. Most if not all properties of 1 can be deduced from this. In advanced mathematics, a multiplicative identity is often denoted 1, even if it is not a number. 1 is by convention not considered a prime number; this was not universally accepted until the mid-20th century. Additionally, 1 is the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Nuclide
Stable nuclides are nuclides that are not radioactive and so (unlike radionuclides) do not spontaneously undergo radioactive decay. When such nuclides are referred to in relation to specific elements, they are usually termed stable isotopes. The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been known to decay using current equipment (see list at the end of this article). Of these 80 elements, 26 have only one stable isotope; they are thus termed monoisotopic. The rest have more than one stable isotope. Tin has ten stable isotopes, the largest number of stable isotopes known for an element. Definition of stability, and naturally occurring nuclides Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 34 more (total of 286) are known to be radioactive with sufficiently long half-lives (also known) to occur primordially. If the half-life of a nuclide is comparable to, or greater t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to the magnetic field. A permanent magnet's magnetic field pulls on ferromagnetic materials such as iron, and attracts or repels other magnets. In addition, a nonuniform magnetic field exerts minuscule forces on "nonmagnetic" materials by three other magnetic effects: paramagnetism, diamagnetism, and antiferromagnetism, although these forces are usually so small they can only be detected by laboratory equipment. Magnetic fields surround magnetized materials, and are created by electric currents such as those used in electromagnets, and by electric fields varying in time. Since both strength and direction of a magnetic field may vary with location, it is described mathematically by a function assigning a vector to each point of space ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field for a system of charged particles. Electric fields originate from electric charges and time-varying electric currents. Electric fields and magnetic fields are both manifestations of the electromagnetic field, one of the four fundamental interactions (also called forces) of nature. Electric fields are important in many areas of physics, and are exploited in electrical technology. In atomic physics and chemistry, for instance, the electric field is the attractive force holding the atomic nucleus and electrons together in atoms. It is also the force responsible for chemical bonding between atoms that result in molecules. The electric field is defined as a vector field that associates to each point in space the electrostatic ( Coulomb) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Velocity Selector
A Wien filter also known as velocity selector is a device consisting of perpendicular electric and magnetic fields that can be used as a velocity filter for charged particles, for example in electron microscopes and spectrometers. It is used in accelerator mass spectrometry to select particles based on their speed. The device is composed of orthogonal electric and magnetic fields, such that particles with the correct speed will be unaffected while other particles will be deflected. It is named for Wilhelm Wien who developed it in 1898 for the study of anode rays. It can be configured as a charged particle energy analyzer, monochromator, or mass spectrometer. Theory Any charged particle in an electric field will feel a force proportional to the charge and field strength such that \vec = q \vec, where ''F'' is force, ''q'' is charge, and ''E'' is electric field strength. Similarly, any particle moving in a magnetic field will feel a force proportional to the velocity and charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sector Instrument
A sector instrument is a general term for a class of mass spectrometer that uses a static electric (E) or magnetic (B) sector or some combination of the two (separately in space) as a mass analyzer. Popular combinations of these sectors have been the EB, BE (of so-called reverse geometry), three-sector BEB and four-sector EBEB (electric-magnetic-electric-magnetic) instruments. Most modern sector instruments are double-focusing instruments (first developed by Francis William Aston, Arthur Jeffrey Dempster, Kenneth Bainbridge and Josef Mattauch in 1936) in that they focus the ion beams both in direction and velocity. Theory The behavior of ions in a homogeneous, linear, static electric or magnetic field (separately) as is found in a sector instrument is simple. The physics are described by a single equation called the Lorentz force law. This equation is the fundamental equation of all mass spectrometric techniques and applies in non-linear, non-homogeneous cases too and is an i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Annual Review Of Nuclear And Particle Science
Annual may refer to: *Annual publication, periodical publications appearing regularly once per year **Yearbook **Literary annual *Annual plant *Annual report *Annual giving *Annual, Morocco, a settlement in northeastern Morocco *Annuals (band), a musical group See also * Annual Review (other) Annual Review or Annual Reviews may refer to: * An annual performance appraisal or performance review of an employee * Annual Reviews (publisher), a publisher of academic journals * The ''Annual Reviews'' series of journals is published by Annual ... * Circannual cycle, in biology {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van De Graaff Generator
A Van de Graaff generator is an electrostatic generator which uses a moving belt to accumulate electric charge on a hollow metal globe on the top of an insulated column, creating very high electric potentials. It produces very high voltage High voltage electricity refers to electrical potential large enough to cause injury or damage. In certain industries, ''high voltage'' refers to voltage above a certain threshold. Equipment and conductors that carry high voltage warrant sp ... direct current (DC) electricity at low current levels. It was invented by American physicist Robert J. Van de Graaff in 1929. The potential difference achieved by modern Van de Graaff generators can be as much as 5 megavolts. A tabletop version can produce on the order of 100 kV and can store enough energy to produce visible electric sparks. Small Van de Graaff machines are produced for entertainment, and for physics education to teach electrostatics; larger ones are displayed in some science muse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Source
An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines. Electron ionization Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is :M + e^- -> M^ + 2e^- where M is the atom or molecule being ionized, e^- is the electron, and M^ is the resulting ion. The electrons may be created by an arc discharge between a cathode and an anode. An electron beam ion source (EBIS) is used in atomic physics to produce highly charged ions by bombarding atoms with a powerful electron beam. Its principle of operation is shared by the electron beam ion trap. Electron capture ionization Electron capture ionization (ECI) is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form A−•. The reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionized
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ionizat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)