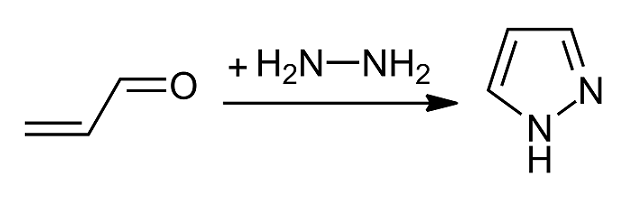|
Azines
Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones. For example, acetone azine is the simplest ketazine. Aldazines are azines derived from aldehydes. Preparation The usual method of industrial production is the peroxide process, starting from the ketone, ammonia, and hydrogen peroxide. : In the laboratory, azines are typically prepared by condensation of hydrazine with two equivalents of a carbonyl. Azines are also produced when chalcone reacts with a hydrazone to produce 3,5-diphenyl-1''H''-pyrazole, in a conversion also carried out with hydrazine hydrate. : Reactions Azines characteristically undergo hydrolysis to hydrazines. The reaction proceeds by the intermediacy of a hydrazone: :R2C=N-N=CR2 + H2O → R2C=N-NH2 + R2C=O :R2C=N-NH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azine
Azines are a functional group, functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones. For example, acetone azine is the simplest ketazine. Aldazines are azines derived from aldehydes. Preparation The usual method of industrial production is the peroxide process, starting from the ketone, ammonia, and hydrogen peroxide. : In the laboratory, azines are typically prepared by condensation of hydrazine with two equivalents of a carbonyl. Azines are also produced when chalcone reacts with a hydrazone to produce 3,5-diphenyl-1''H''-pyrazole, in a conversion also carried out with hydrazine hydrate. : Reactions Azines characteristically undergo hydrolysis to hydrazines. The reaction proceeds by the intermediacy of a hydrazone: :R2C=N-N=CR2 + H2O → R2C=N-NH2 + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine Hydrate
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. Synthesis Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones. : Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides. Uses Hydrazones are the basis for various analyses of ketones and aldehydes. For example, dinitrophenylhydrazine coated onto a silica sorbent is the basis of an adsorption cartridge. The hydrazones are then eluted and analyzed by HPLC using a UV detector. The compound carbonyl cyanide-''p''-trifluoromethoxyphenylhydrazone (abbreviated as FCCP) is used to uncouple ATP synthesis and reduction of oxygen in oxidative phosphorylation in mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone Azine
Acetone azine is the simplest ketazine. It is an intermediate in some hydrazine manufacturing processes. Synthesis Acetone azine can be prepared from acetone and hydrazine: :2 (CH3)2CO + N2H4 → 2 H2O + CH3)2C=Nsub>2 It can also be produced from acetone (2 eq.), ammonia (2 eq.) and hydrogen peroxide (1 eq.). The first step is the formation of acetone imine, Me2C=NH; this is then oxidized by hydrogen peroxide through a complex mechanism to give 3,3-dimethyloxaziridine, which reacts with a further molecule of ammonia to produce acetone hydrazone. The hydrazone then condenses with a further molecule of acetone to produce the azine. The acetone azine product is distilled out of the reaction mixture as its azeotrope with water (''n''(H2O)/''n''(azine) ≈ 6). Reactions Acetone azine can be used to prepare acetone hydrazone Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide Process
The peroxide process is a method for the industrial production of hydrazine. In this process hydrogen peroxide is used as an oxidant instead of sodium hypochlorite, which is traditionally used to generate hydrazine. The main advantage of the peroxide process to hydrazine relative to the traditional Olin Raschig process is that it does not coproduce salt. In this respect, the peroxide process is an example of green chemistry. Since many millions of kilograms of hydrazine are produced annually, this method is of both commercial and environmental significance.Jean-Pierre Schirmann, Paul Bourdauducq "Hydrazine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. . Production Ketazine formation In the usual implementation, hydrogen peroxide is used together with acetamide. This mixture does not react with ammonia directly but does so in the presence of methyl ethyl ketone to give the oxaziridine. : Balanced equations for the individual steps are as follows. Imi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine ( Knorr-type reactions). For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole: :CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O History The term pyrazole was given to this class of compounds by German ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture. Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Gas chromatography is the process of separating compounds in a mixture by injecting a gaseous or liquid sample into a mobile phase, typically called the carrier gas, and passing the gas through a stationary phase. The mobile phase is usually an inert gas or an unreactive gas such as helium, argon, nitrogen or hydrogen. The stationary phase is a microscopic lay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Chemistry (journal)
''Analytical Chemistry'' is a biweekly peer-reviewed scientific journal published since 1929 by the American Chemical Society. Articles address general principles of chemical measurement science and novel analytical methodologies. Topics commonly include chemical reactions and selectivity, chemometrics and data processing, electrochemistry, elemental and molecular characterization, imaging, instrumentation, mass spectrometry, microscale and nanoscale systems, -omics, sensing, separations, spectroscopy, and surface analysis. It is abstracted and indexed in Chemical Abstracts Service, CAB International, EBSCOhost, ProQuest, PubMed, Scopus, and the Science Citation Index Expanded. According to the ''Journal Citation Reports'', it has a 2020 impact factor of 6.986 . The editor-in-chief is Jonathan V. Sweedler (University of Illinois). See also *List of chemistry journals References External links * {{DEFAULTSORT:Analytical Chemistry (Journal) Analytical Chemistry Analytical c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an Redox, oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page for EPA reports on pesticide use ihere Selective herbicides control specific weed species, while leaving the desired crop relatively unharmed, while non-selective herbicides (sometimes called total weedkillers in commercial products) can be used to clear waste ground, industrial and construction sites, railways and railway embankments as they kill all plant material with which they come into contact. Apart from selective/non-selective, other important distinctions include ''persistence'' (also known as ''residual action'': how long the product stays in place and remains active), ''means of uptake'' (whether it is absorbed by above-ground foliage only, through the roots, or by other means), and ''mechanism of action'' (how it works). Histo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |