|
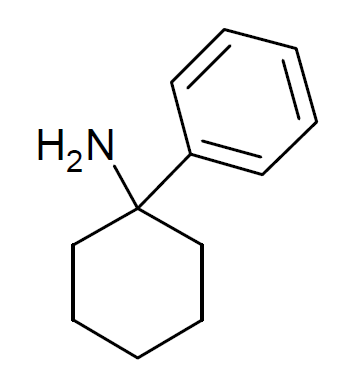
Arylcyclohexylamine
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCP itself was discovered in 1926 but not researched by the pharmaceutical industry until the 1950s. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 studies of PCP and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Eticyclidine
PCE (Eticyclidine, CI-400) is a dissociative anesthetic drug with hallucinogenic effects. It is similar in effects to phencyclidine but is slightly more potent. PCE was developed by Parke-Davis in the 1970s and evaluated for anesthetic potential under the code name CI-400, but research into PCE was not continued after the development of ketamine, a similar drug with more favourable properties. Due to its similarity in effects to PCP, PCE was placed into the Schedule 1 list of illegal drugs in the 1970s, although it was only briefly abused in the 1970s and 1980s and is now little known. See also * Arylcyclohexylamine * 3-MeO-PCE * 3-MeO-PCP * 4-MeO-PCP * Phencyclidine * PCPr * Methoxetamine Methoxetamine (MXE) is a dissociative hallucinogen that has been sold as a designer drug. It differs from many dissociatives such as ketamine and phencyclidine (PCP) that were developed as pharmaceutical drugs for use as general anesthetics in ... References Arylcyclohex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phencyclidine Structure
Phencyclidine or phenylcyclohexyl piperidine (PCP), also known in its use as a Recreational drug use, street drug as angel dust among other names, is a dissociative anesthetic mainly recreational drug use, used recreationally for its significant mind-altering effects. PCP may cause hallucinations, distorted perceptions of sounds, and psychosis, psychotic behavior. As a recreational drug, it is typically Smoking, smoked, but may be taken Oral administration, by mouth, Insufflation (medicine), snorted, or Injection (medicine), injected. It may also be mixed with Cannabis (drug), cannabis or tobacco. Adverse effects may include paranoia, addiction, and an increased risk of suicide, as well as seizures and coma in cases of overdose. Hallucinogen persisting perception disorder, Flashbacks may occur despite stopping usage. Chemically, PCP is a member of the arylcyclohexylamine chemical classification, class. PCP works primarily as an NMDA receptor antagonist. PCP is most commonly u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phencyclidine
Phencyclidine or phenylcyclohexyl piperidine (PCP), also known in its use as a street drug as angel dust among other names, is a dissociative anesthetic mainly used recreationally for its significant mind-altering effects. PCP may cause hallucinations, distorted perceptions of sounds, and psychotic behavior. As a recreational drug, it is typically smoked, but may be taken by mouth, snorted, or injected. It may also be mixed with cannabis or tobacco. Adverse effects may include paranoia, addiction, and an increased risk of suicide, as well as seizures and coma in cases of overdose. Flashbacks may occur despite stopping usage. Chemically, PCP is a member of the arylcyclohexylamine class. PCP works primarily as an NMDA receptor antagonist. PCP is most commonly used in the US. While usage peaked in the US in the 1970s, between 2005 and 2011, an increase in visits to emergency departments as a result of the drug occurred. As of 2022, in the US, about 0.7% of 12th-grade ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dissociative
Dissociatives, colloquially dissos, are a subclass of hallucinogens that distort perception of sight and sound and produce feelings of detachment – dissociation – from the environment and/or self. Although many kinds of drugs are capable of such an effect, dissociatives are unique in that they do so in such a way that they produce hallucinogenic effects, which may include dissociation, a general decrease in sensory experience, hallucinations, dream-like states or anesthesia. Despite most dissociatives' main mechanism of action being tied to NMDA receptor antagonism, some of these substances, which are nonselective in action and affect the dopamine and/or opioid systems, may be capable of inducing more ''direct'' and repeatable euphoria or symptoms which are more akin to the effects of typical " hard drugs" or common drugs of abuse. This is likely why dissociatives are considered to be addictive with a fair to moderate potential for abuse, unlike psychedelics. Despite s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ketamine
Ketamine is a cyclohexanone-derived general anesthetic and NMDA receptor antagonist with analgesic and hallucinogenic properties, used medically for anesthesia, depression, and pain management. Ketamine exists as its S- (esketamine) and R- (arketamine) two enantiomers and has antidepressant action likely involving additional mechanisms than NMDA antagonism. At anesthetic doses, ketamine induces a state of dissociative anesthesia, a trance-like state providing pain relief, sedation, and amnesia. Its distinguishing features as an anesthestic are preserved breathing and airway reflexes, stimulated heart function with increased blood pressure, and moderate bronchodilation. As an anesthetic, it is used especially in trauma, Emergency medical services, emergency, and Pediatrics, pediatric cases. At lower, sub-anesthetic doses, it is used as a treatment for pain and treatment-resistant depression. Ketamine is legally used in medicine but is also tightly controlled due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, typical of amines. The name comes from the genus name '' Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Primary Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phenyl Ring
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen atom, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with and is represented by the symbol Ph (archaically, Φ), or Ø. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. Fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of matter and its properties. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms. Chemists carefully measure substance proportions, chemical reaction rates, and other chemical properties. In Commonwealth English, pharmacists are often called chemists. Chemists use their knowledge to learn the composition and properties of unfamiliar substances, as well as to reproduce and synthesize large quantities of useful naturally occurring substances and create new artificial substances and useful processes. Chemists may specialize in any number of Chemistry#Subdisciplines, subdisciplines of chemistry. Materials science, Materials scientists and metallurgists sha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Geminal
In chemistry, the descriptor geminal () refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol (a molecule that has two alcohol functional groups) attached to the same carbon atom, as in methanediol. Also the shortened prefix ''gem'' may be applied to a chemical name to denote this relationship, as in a ''gem''-dibromide for "geminal dibromide". The concept is important in many branches of chemistry, including synthesis and spectroscopy, because functional groups attached to the same atom often behave differently from when they are separated. Geminal diols, for example, are easily converted to ketones or aldehydes with loss of water.Peter Taylor (2002)''Mechanism and synthesis'' Book 10 of ''Molecular world''. Open University, Royal Society of Chemistry; . 368 pages The related term '' vicinal'' refers to the relationship between two functional groups that are attached to adjacent atoms. Thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known. Production and synthesis Industrial production Pyrrolidine is prepared industrially by the reaction of 1,4-butanediol and ammonia at a temperature of 165–200 °C and a pressure of 17–21 MPa in the presence of a cobalt- and nickel oxide catalyst, which is supported on alumina. : The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode. The product is obtained after m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |