|
Apoaequorin
Aequorin is a calcium-activated photoprotein isolated from the hydrozoan ''Aequorea victoria''. Its bioluminescence was studied decades before the protein was isolated from the animal by Osamu Shimomura in 1962. In the animal, the protein occurs together with the green fluorescent protein to produce green light by resonant energy transfer, while aequorin by itself generates blue light. Discussions of "jellyfish DNA" that can make "glowing" animals often refer to transgenic animals that express the green fluorescent protein, not aequorin, although both originally derive from the same animal. Apoaequorin, the protein portion of aequorin, is an ingredient in the dietary supplement Prevagen. The US Federal Trade Commission (FTC) has charged the maker with false advertising for its memory improvement claims. Discovery Work on aequorin began with E. Newton Harvey in 1921. Though Harvey was unable to demonstrate a classical luciferase-luciferin reaction, he showed that water cou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EF Hand
The EF hand is a helix–loop–helix structural domain or ''motif'' found in a large family of calcium-binding proteins. The EF-hand motif contains a helix–loop–helix topology, much like the spread thumb and forefinger of the human hand, in which the Ca2+ ions are coordinated by ligands within the loop. The motif takes its name from traditional nomenclature used in describing the protein parvalbumin, which contains three such motifs and is probably involved in muscle relaxation via its calcium-binding activity. The EF-hand consists of two alpha helices linked by a short loop region (usually about 12 amino acids) that usually binds calcium ions. EF-hands also appear in each structural domain of the signaling protein calmodulin and in the muscle protein troponin-C. Calcium ion binding site The calcium ion is coordinated in a pentagonal bipyramidal configuration. The six residues involved in the binding are in positions 1, 3, 5, 7, 9 and 12; these residues are denoted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photocytes
A photocyte is a cell that specializes in catalyzing enzymes to produce light (bioluminescence). Photocytes typically occur in select layers of epithelial tissue, functioning singly or in a group, or as part of a larger apparatus (a photophore). They contain special structures called photocyte granules. These specialized cells are found in a range of multicellular animals, including coelenterates (cnidarians and ctenophores), annelids, arthropods (including insects) and fishes. Although some fungi are bioluminescent, they do not have such specialized cells. Mechanism of light production Nerve impulses may first trigger light production by stimulating the photocyte to release the enzyme luciferase into a "reaction chamber" of luciferin substrate. In some species, the release occurs continually without the precursor impulse via osmotic diffusion. Molecular oxygen is then actively gated through surrounding tracheal cells which otherwise limit the natural diffusion of oxygen from bl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
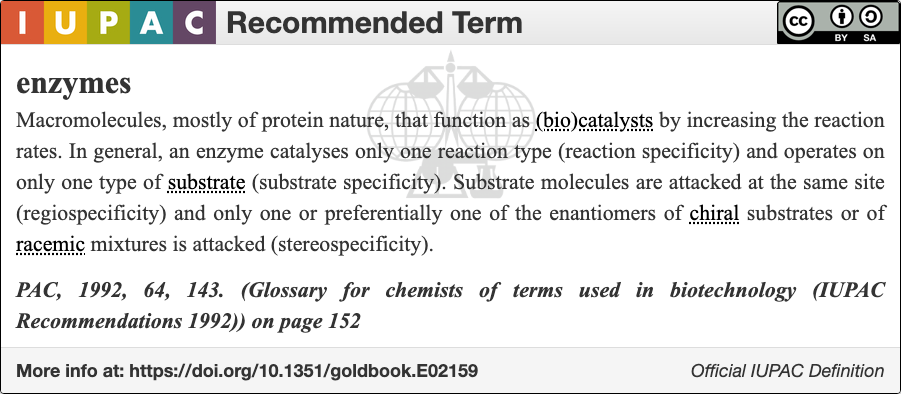
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coelenterazine
Coelenterazine is a luciferin, a molecule that emits light after reaction with oxygen, found in many aquatic organisms across eight phyla. It is the substrate of many luciferases such as '' Renilla reniformis'' luciferase (Rluc), ''Gaussia'' luciferase (Gluc), and photoproteins, including aequorin, and obelin. All these proteins catalyze the oxidation of this substance, a reaction catalogued EC 1.13.12.5. History Coelenterazine was simultaneously isolated and characterized by two groups studying the luminescent organisms sea pansy ('' Renilla reniformis'') and the cnidarian ''Aequorea victoria'', respectively. Both groups independently discovered that the same compound was used in both luminescent systems. The molecule was named after the now-obsolete phylum coelenterata. Likewise, the two main metabolites – coelenteramide and coelenteramine – were named after their respective functional groups. While coelenterazine was first discovered in ''Aequorea victoria'', it wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prosthetic Group
A prosthetic group is the non-amino acid component that is part of the structure of the heteroproteins or conjugated proteins, being tightly linked to the apoprotein. Not to be confused with the cosubstrate that binds to the enzyme apoenzyme (either a holoprotein or heteroprotein) by non-covalent binding a non-protein (non-amino acid) This is a component of a conjugated protein that is required for the protein's biological activity. The prosthetic group may be organic (such as a vitamin, sugar, RNA, phosphate or lipid) or inorganic (such as a metal ion). Prosthetic groups are bound tightly to proteins and may even be attached through a covalent bond. They often play an important role in enzyme catalysis. A protein without its prosthetic group is called an apoprotein, while a protein combined with its prosthetic group is called a holoprotein. A non-covalently bound prosthetic group cannot generally be removed from the holoprotein without denaturating the protein. Thus, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Mass Unit
The dalton or unified atomic mass unit (symbols: Da or u, respectively) is a unit of mass defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. It is a non-SI unit accepted for use with SI. The word "unified" emphasizes that the definition was accepted by both IUPAP and IUPAC. The atomic mass constant, denoted , is defined identically. Expressed in terms of , the atomic mass of carbon-12: . Its value in SI units is an experimentally determined quantity. The 2022 CODATA recommended value of the atomic mass constant expressed in the SI base unit kilogram is:This value serves as a conversion factor of mass from daltons to kilograms, which can easily be converted to grams and other metric units of mass. The 2019 revision of the SI redefined the kilogram by fixing the value of the Planck constant (), improving the precision of the atomic mass constant expressed in SI units by anchoring it to fixed physica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoenzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include catalytic RNA molecules, also called ribozymes. They are sometimes described as a ''type'' of enzyme rather than being ''like'' an enzyme, but even in the deca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holoprotein
A holoprotein or conjugated protein is an apoprotein combined with its prosthetic group. Some enzymes do not need additional components to show full activity. Others require non-protein molecules called cofactors to be bound for activity. Cofactors can be either inorganic (e.g., metal ions and iron-sulfur clusters) or organic compounds (e.g., flavin and heme). Organic cofactors can be either coenzymes, which are released from the enzyme's active site during the reaction, or prosthetic groups, which are tightly bound to an enzyme. Organic prosthetic groups can be covalently bound (e.g., biotin in enzymes such as pyruvate carboxylase). An example of an enzyme that contains a cofactor is carbonic anhydrase, which has a zinc cofactor bound as part of its active site. These tightly bound ions or molecules are usually found in the active site and are involved in catalysis. For example, flavin and heme cofactors are often involved in redox reactions. Enzymes that require a cofactor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobel Prize
The Nobel Prizes ( ; ; ) are awards administered by the Nobel Foundation and granted in accordance with the principle of "for the greatest benefit to humankind". The prizes were first awarded in 1901, marking the fifth anniversary of Alfred Nobel, Alfred Nobel's death. The original Nobel Prizes covered five fields: Nobel Prize in Physics, physics, Nobel Prize in Chemistry, chemistry, Nobel Prize in Physiology or Medicine, physiology or medicine, Nobel Prize in Literature, literature, and Nobel Peace Prize, peace, specified in Nobel's will. A sixth prize, the Nobel Memorial Prize in Economic Sciences, Prize in Economic Sciences, was established in 1968 by Sveriges Riksbank (Sweden's central bank) in memory of Alfred Nobel. The Nobel Prizes are widely regarded as the most prestigious awards available in their respective fields.Nobel Prize#Shalev69, Shalev, p. 8. Except in extraordinary circumstances, such as war, all six prizes are given annually. Each recipient, known as a laur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Roger Tsien
Roger Yonchien Tsien (Chinese: 錢永健'';'' February 1, 1952 – August 24, 2016) was an American biochemist. He was a professor of chemistry and biochemistry at the University of California, San Diego, and was awarded the Nobel Prize in Chemistry in 2008 for his discovery and development of the green fluorescent protein, in collaboration with organic chemist Osamu Shimomura and neurobiologist Martin Chalfie. Tsien was also a pioneer of calcium imaging. Early life Tsien was born to a Chinese American family in New York in 1952. He grew up in Livingston, New Jersey and attended Livingston High School. Tsien traces his family ancestry to Hangzhou, China. His father Hsue-Chu Tsien, an MIT and Shanghai Chiao Tung University alumnus, was a mechanical engineer and had excelled academically, graduating at the top of his university class. Tsien suffered from asthma as a child, and as a result, he was often indoors. He spent hours conducting chemistry experiments in his b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martin Chalfie
Martin Lee Chalfie (born January 15, 1947) is an American scientist. He is University Professor at Columbia University. He shared the 2008 Nobel Prize in Chemistry along with Osamu Shimomura and Roger Y. Tsien "for the discovery and development of the green fluorescent protein, GFP". He holds a PhD in neurobiology from Harvard University. Education and early life Chalfie grew up in Chicago, Illinois, son of the guitarist Eli Chalfie (1910–1996) and owner of an apparel store Vivian Chalfie (née Friedlen, 1913–2005). His maternal grandfather, Meyer L. Friedlen, immigrated to Chicago from Moscow at an early age; his paternal grandparents, Benjamin and Esther Chalfie, came to Cincinnati from Brest-Litovsk and are Jewish. He matriculated at Harvard University in 1965, intending to be a math major, but he switched to biochemistry because it combined his interests in chemistry, math, and biology. He spent the summer after his junior year working in the laboratory of Kla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |