|
Antimony Electrode
The antimony electrode has been investigated for its ability to function as a pH electrode.Bates, Roger G. ''Determination of pH: theory and practice''. Wiley, 1973, pp 300-304 The electrode is made of elemental antimony. The electrochemical process can be formulated as :Sb2O3 + 6 H+ + 6 e- 2Sb + 3 H2O The oxide, Sb2O3, is present on the surface of the electrode. Although this electrode does not give measurements of high accuracy, its rapid response, simplicity and rugged construction make it useful for continuous industrial pH monitoring. It can be used at elevated temperatures. In an unusual application, an antimony electrode was used to measure pH inside the human stomach. The simplicity of construction meant that the electrode could be made small enough to be swallowed. Thin copper wires were attached to the electrode and one terminal on a pH meter. The subject's foot was placed in a saline solution. A calomel reference electrode was also placed in this solution and was connec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name Kohl (cosmetics), kohl. The earliest known description of this metalloid in the West was written in 1540 by Vannoccio Biringuccio. China is the largest producer of antimony and its compounds, with most production coming from the Xikuangshan Mine in Hunan. The industrial methods for refining antimony from stibnite are Roasting (metallurgy), roasting followed by carbothermic reaction, reduction with carbon, or direct reduction of stibnite with iron. The most common applications for metallic antimony are in alloys with lead and tin, which have improved properties for solders, Bullet, bullets, and plain bearings. It improves the rigidity of lead-alloy pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling point near room temperature. It is used to make most organofluorine compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. The most common application of ion-selective glass electrodes is for the measurement of pH. The pH electrode is an example of a glass electrode that is sensitive to hydrogen ions. Glass electrodes play an important part in the instrumentation for chemical analysis, and physicochemical studies. The voltage of the glass electrode, relative to some reference value, is sensitive to changes in the activity of certain types of ions. History The first studies of glass electrodes (GE) found different sensitivities of different glasses to change the medium's acidity ( pH), due to the effects of the alkali metal ions. In 1906, M. Cremer, the father of Erika Cremer, determined that the electric potential that arises between parts of the fluid, located on opposite sides of the glass membrane, is proportional to the concentration of acid (hydrogen ion concentration). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
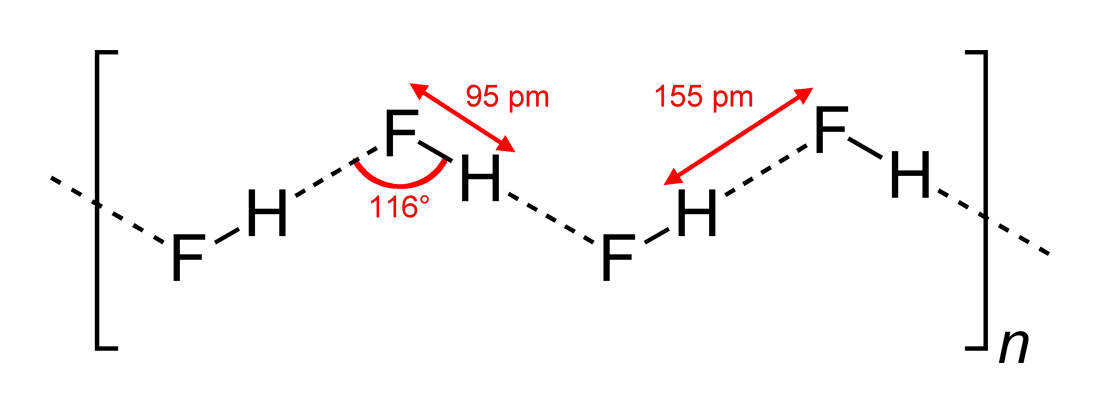
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrodes
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes. Michael Faraday coined the term "" in 1833; the word recalls the Greek ἤλεκτρον (, "amber") and ὁδός (, "path, way"). The electrophore, invented by Johan Wilcke in 1762, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes sep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |