|
Antihelium
In modern physics, antimatter is defined as matter composed of the antiparticles (or "partners") of the corresponding particles in "ordinary" matter, and can be thought of as matter with reversed charge and parity, or going backward in time (see CPT symmetry). Antimatter occurs in natural processes like cosmic ray collisions and some types of radioactive decay, but only a tiny fraction of these have successfully been bound together in experiments to form antiatoms. Minuscule numbers of antiparticles can be generated at particle accelerators, but total artificial production has been only a few nanograms. No macroscopic amount of antimatter has ever been assembled due to the extreme cost and difficulty of production and handling. Nonetheless, antimatter is an essential component of widely available applications related to beta decay, such as positron emission tomography, radiation therapy, and industrial imaging. In theory, a particle and its antiparticle (for example, a proto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
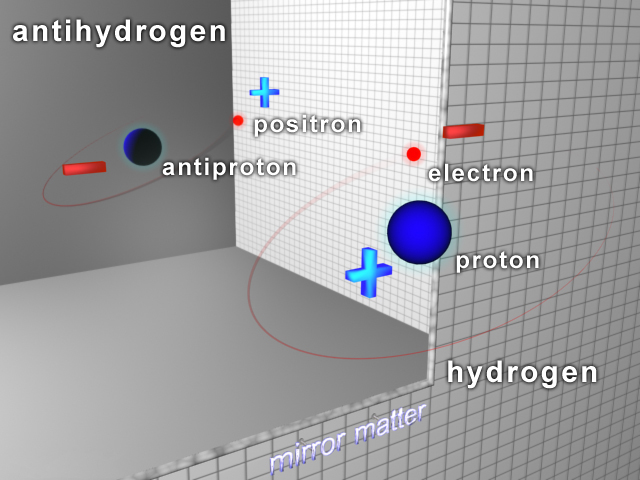
Antihydrogen
Antihydrogen () is the antimatter counterpart of hydrogen. Whereas the common hydrogen atom is composed of an electron and proton, the antihydrogen atom is made up of a positron and antiproton. Scientists hope that studying antihydrogen may shed light on the question of why there is more matter than antimatter in the observable universe, known as the baryon asymmetry problem. Antihydrogen is produced artificially in particle accelerators. Experimental history Accelerators first detected hot antihydrogen in the 1990s. ATHENA studied cold in 2002. It was first trapped by the Antihydrogen Laser Physics Apparatus (ALPHA Collaboration, ALPHA) team at CERN in 2010, who then measured the structure and other important properties. ALPHAAEgIS and GBAR plan to further cool and study atoms. 1s–2s transition measurement In 2016, the Antiproton Decelerator#ALPHA, ALPHA experiment measured the atomic electron transition between the two lowest energy levels of antihydrogen, 1s–2s. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmic Ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our own galaxy, and from distant galaxies. Upon impact with Earth's atmosphere, cosmic rays produce showers of secondary particles, some of which reach the surface, although the bulk are deflected off into space by the magnetosphere or the heliosphere. Cosmic rays were discovered by Victor Hess in 1912 in balloon experiments, for which he was awarded the 1936 Nobel Prize in Physics. Direct measurement of cosmic rays, especially at lower energies, has been possible since the launch of the first satellites in the late 1950s. Particle detectors similar to those used in nuclear and high-energy physics are used on satellites and space probes for research into cosmic rays. Data from the Fermi Space Telescope (2013) have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positron
The positron or antielectron is the particle with an electric charge of +1''elementary charge, e'', a Spin (physics), spin of 1/2 (the same as the electron), and the same Electron rest mass, mass as an electron. It is the antiparticle (antimatter counterpart) of the electron. When a positron collides with an electron, annihilation occurs. If this collision occurs at low energies, it results in the production of two or more photons. Positrons can be created by positron emission radioactive decay (through weak interactions), or by pair production from a sufficiently energetic photon which is interacting with an atom in a material. History Theory In 1928, Paul Dirac published a paper proposing that electrons can have both a positive and negative charge. This paper introduced the Dirac equation, a unification of quantum mechanics, special relativity, and the then-new concept of electron Spin (physics), spin to explain the Zeeman effect. The paper did not explicitly predict a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Number
In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. To fully specify the state of the electron in a hydrogen atom, four quantum numbers are needed. The traditional set of quantum numbers includes the principal, azimuthal, magnetic, and spin quantum numbers. To describe other systems, different quantum numbers are required. For subatomic particles, one needs to introduce new quantum numbers, such as the flavour of quarks, which have no classical correspondence. Quantum numbers are closely related to eigenvalues of observables. When the corresponding observable commutes with the Hamiltonian of the system, the quantum number is said to be " good", and acts as a constant of motion in the quantum dynamics. History Electronic quantum numbers In the era of the old quantum theory, starting from Max Planck's proposal of quanta in his model of blackbody radiation (1900) and Albert Einstein's adaptation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features Peer review, peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2022 ''Journal Citation Reports'' (with an ascribed impact factor of 50.5), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in the autumn of 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander MacMillan (publisher), Alexander MacMillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester based on the 1909 Geiger–Marsden experiments, Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass–energy Equivalence
In physics, mass–energy equivalence is the relationship between mass and energy in a system's rest frame. The two differ only by a multiplicative constant and the units of measurement. The principle is described by the physicist Albert Einstein's formula: E = mc^2. In a reference frame where the system is moving, its relativistic energy and relativistic mass (instead of rest mass) obey the same formula. The formula defines the energy () of a particle in its rest frame as the product of mass () with the speed of light squared (). Because the speed of light is a large number in everyday units (approximately ), the formula implies that a small amount of mass corresponds to an enormous amount of energy. Rest mass, also called invariant mass, is a fundamental physical property of matter, independent of velocity. Massless particles such as photons have zero invariant mass, but massless free particles have both momentum and energy. The equivalence principle implies that w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionizing Radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum. Gamma rays, X-rays, and the higher energy vacuum ultraviolet, ultraviolet part of the electromagnetic spectrum are ionizing radiation; whereas the lower energy ultraviolet, visible light, infrared, microwaves, and radio waves are non-ionizing radiation. Nearly all types of laser light are non-ionizing radiation. The boundary between ionizing and non-ionizing radiation in the ultraviolet area cannot be sharply defined, as different molecules and atoms ionize at Ionization energies of the elements (data page), different energies. The energy of ionizing radiation starts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |