|
Antibody Mimetic
Antibody mimetics are organic compounds that, like antibodies, can specifically bind antigens, but that are not structurally related to antibodies. They are usually artificial peptides or proteins with a molar mass of about 3 to 20 kDa. (Antibodies are ~150 kDa.) Nucleic acids and small molecules are sometimes considered antibody mimetics as well, but not artificial antibodies, Fragment antigen-binding, antibody fragments and fusion proteins composed from these. Common advantages over antibodies are better solubility, tissue penetration, stability towards heat and enzymes, and comparatively low production costs. Antibody mimetics are being developed as therapeutic and Medical diagnosis, diagnostic agents. Examples See also *Protein mimetic *Optimer Ligand References {{Engineered antibodies Antibody mimetics, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibodies
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as bacteria and viruses, including those that cause disease. Each individual antibody recognizes one or more specific antigens, and antigens of virtually any size and chemical composition can be recognized. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each of the branching chains comprising the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" the antigen (or a microbe or an infected cell bearing such an antigen) for attack by cells of the immune system, or can neutralize it directly (for example, by blocking a part of a viru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affimers
Affimer molecules are small proteins that bind to target proteins with affinity in the nanomolar range. These engineered non-antibody binding proteins are designed to mimic the molecular recognition characteristics of monoclonal antibodies in different applications. These affinity reagents have been optimized to increase their stability, make them tolerant to a range of temperatures and pH, reduce their size, and to increase their expression in ''E.coli'' and mammalian cells. Development Affimer proteins were developed initially at the MRC Cancer Cell Unit in Cambridge then across two laboratories at the University of Leeds. Derived from the cysteine protease inhibitor family of cystatins, which function in nature as cysteine protease inhibitors, these 12–14 kDa proteins share the common tertiary structure of an alpha-helix lying on top of an anti-parallel beta-sheet. Affimer proteins display two peptide loops that can all be randomized to bind to desired target proteins, in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ankyrin Repeat
The ankyrin repeat is a 33-residue motif in proteins consisting of two alpha helices separated by loops, first discovered in signaling proteins in yeast Cdc10 and '' Drosophila'' Notch. Domains consisting of ankyrin tandem repeats mediate protein–protein interactions and are among the most common structural motifs in known proteins. They appear in bacterial, archaeal, and eukaryotic proteins, but are far more common in eukaryotes. Ankyrin repeat proteins, though absent in most viruses, are common among poxviruses. Most proteins that contain the motif have four to six repeats, although its namesake ankyrin contains 24, and the largest known number of repeats is 34, predicted in a protein expressed by '' Giardia lamblia''. Ankyrin repeats typically fold together to form a single, linear solenoid structure called ankyrin repeat domains. These domains are one of the most common protein–protein interaction platforms in nature. They occur in a large number of functionall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DARPin
DARPins (an acronym for designed ankyrin repeat proteins) are genetically engineered antibody mimetic proteins typically exhibiting highly specific and high-affinity target protein binding. They are derived from natural ankyrin repeat proteins, one of the most common classes of binding proteins in nature, which are responsible for diverse functions such as cell signaling, regulation and structural integrity of the cell. DARPins consist of at least three repeat motifs or modules, of which the most N- and the most C-terminal modules are referred to as "caps", since they shield the hydrophobic core of the protein. The number of internal modules is indicated as number (e.g. N1C, N2C, N3C, ...) while the caps are indicated with "N" or "C", respectively. The molecular mass of e.g. 14 or 18 kDa (kilodaltons) for four- (N2C) or five- (N3C) repeat DARPins is rather small for a biologic (ca 10% of the size of an IgG). DARPins constitute a new class of potent, specific and versatile smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or Disulfide bond, disulfide bridges. Domains often form functional units, such as the calcium-binding EF-hand, EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera (protein), chimeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Avimer
Avimers (short for ''avidity multimers'') are artificial proteins that are able to specifically bind to certain antigens via multiple binding sites. Since they are not structurally related to antibodies, they are classified as a type of antibody mimetic. Avimers have been developed by the biotechnology company Avidia, now part of Amgen, as potential new pharmaceutical drugs. Structure Avimers consist of two or more peptide sequences of 30 to 35 amino acids each, connected by linker peptides. The individual sequences are derived from A domains of various membrane receptors and have a rigid structure, stabilised by disulfide bonds and calcium. Each A domain can bind to a certain epitope of the target protein. The combination of domains binding to different epitopes of the same protein increases affinity to this protein, an effect known as avidity (hence the name). Alternatively, the domains can be directed against epitopes on different target proteins. This approach is similar to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipocalin
The lipocalins are a family of proteins which transport small hydrophobic molecules such as steroids, bilins, retinoids, and lipids, and most lipocalins are also able to bind to complexed iron (via siderophores or flavonoids) as well as heme. They share limited regions of sequence homology and a common tertiary structure architecture. This is an eight stranded antiparallel beta barrel with a repeated + 1 topology enclosing an internal ligand binding site. These proteins are found in gram negative bacteria, vertebrate cells, and invertebrate cells, and in plants. Lipocalins have been associated with many biological processes, among them immune response, pheromone transport, biological prostaglandin synthesis, retinoid binding, and cancer cell interactions. Function Immune response Lipocalin proteins are important key players of nutritional immunity by withholding and sequestering micronutrients. They are thereby able to regulate inflammatory and detoxification processe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticalin
Anticalin proteins are artificial proteins that are able to bind to antigens, either to proteins or to small molecules. They are not structurally related to antibodies, which makes them a type of antibody mimetic. Instead, they are derived from human lipocalins which are a family of naturally binding proteins. Anticalin proteins are being used in lieu of monoclonal antibodies, but are about eight times smaller with a size of about 180 amino acids and a mass of about 20 kDa. The Anticalin technology is exclusively commercialized by Pieris Pharmaceuticals in Freising, Germany. Anticalin is a registered trademark of Pieris. __TOC__ Properties Anticalin proteins have better tissue penetration than antibodies and are stable at temperatures up to 70 °C. Unlike antibodies, they can be produced in bacterial cells like ''E. coli'' in large amounts. While antibodies can only be directed at macromolecules such as proteins and at small molecules (haptens) only if bound to macromole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
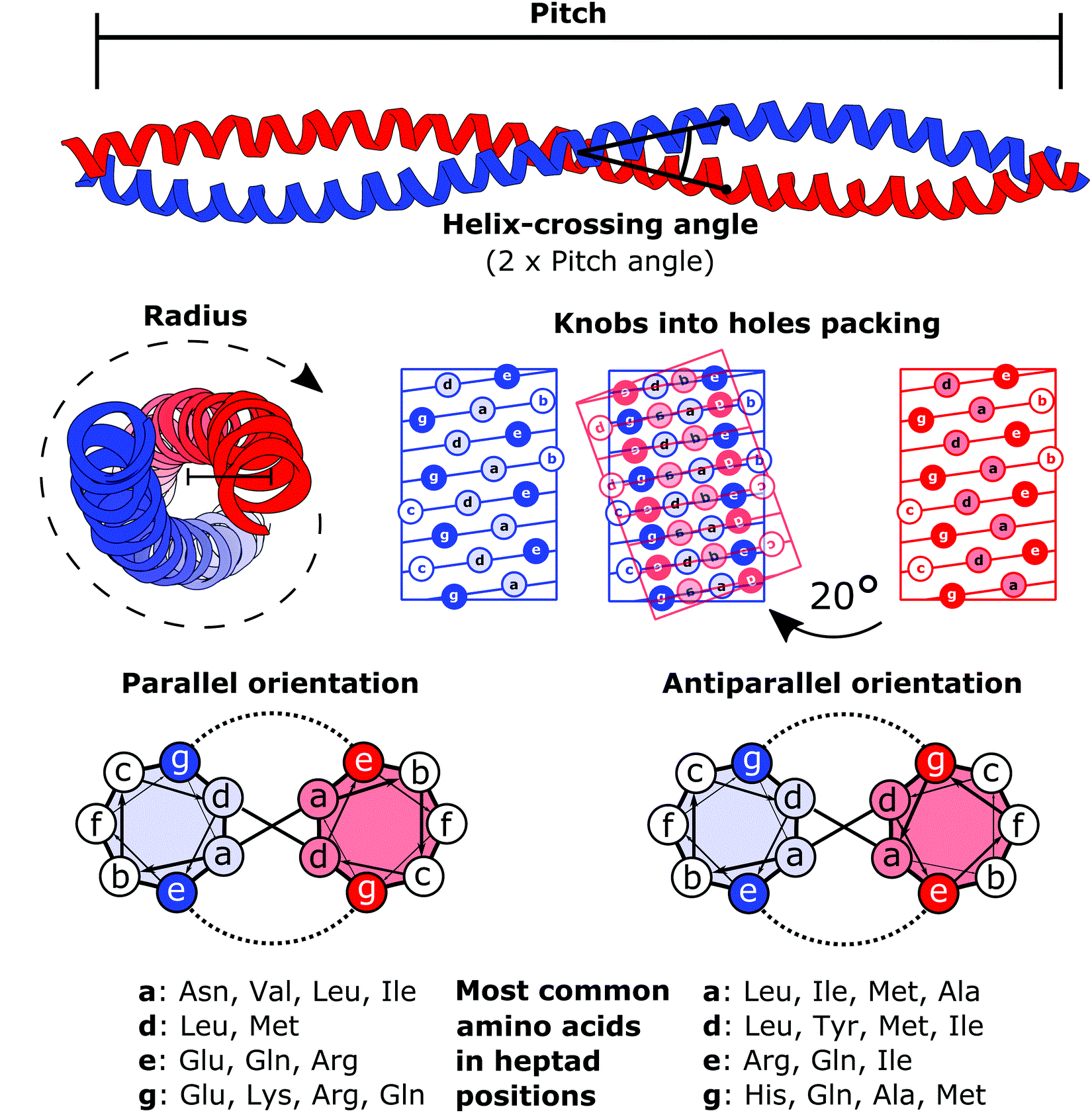
Coiled Coil
A coiled coil is a structural motif in proteins in which two to seven alpha-helices are coiled together like the strands of a rope. ( Dimers and trimers are the most common types.) They have been found in roughly 5-10% of proteins and have a variety of functions. They are one of the most widespread motifs found in protein-protein interactions. To aid protein study, several tools have been developed to predict coiled-coils in protein structures. Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin. Discovery The possibility of coiled coils for α-keratin was initially somewhat controversial. Linus Pauling and Francis Crick independently came to the conclusion that this was possible at about the same time. In the summer of 1952, Pauling visited the laboratory in England where C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alphabody
Alphabodies, also known as Cell-Penetrating Alphabodies or CPAB for short, are small 10 kDa proteins engineered to bind to a variety of antigens. Despite their name, they are not structurally similar to antibodies, which makes them a type of antibody mimetic. Alphabodies are different from many other antibody mimetics in their ability to reach and bind to intracellular protein targets. Their single chain alpha-helical structure is designed by computer modelling, inspired by naturally existing coiled-coil protein structures. Alphabodies are being developed by the Belgium, Belgian biotechnology company Complix N.V. as potential new pharmaceutical drugs against cancer and autoimmune disease. In 2012, a collaboration agreement was signed with Monsanto to develop the technology for agriculture, agricultural applications as well. Development Alphabodies are developed as scaffolds with a set of amino acid residues that can be modified to bind protein targets, while maintaining correct fol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfolobus
''Sulfolobus'' is a genus of microorganism in the family Sulfolobaceae. It belongs to the kingdom Thermoproteati of the Archaea domain. ''Sulfolobus'' species grow in volcanic springs with optimal growth occurring at pH 2–3 and temperatures of 75–80 °C, making them acidophiles and thermophiles respectively. ''Sulfolobus'' cells are irregularly shaped and flagellar. Species of ''Sulfolobus'' are generally named after the location from which they were first isolated, e.g. ''Sulfolobus solfataricus'' (now recombined as ''Saccharolobus solfataricus)'' was first isolated in the Solfatara volcano. Other species can be found throughout the world in areas of volcanic or geothermal activity, such as geological formations called mud pots, which are also known as ''solfatare'' (plural of solfatara). ''Sulfolobus'' as a model to study the molecular mechanisms of DNA replication When the first Archaeal genome, '' Methanococcus jannaschii'', had been sequenced completely i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |