|
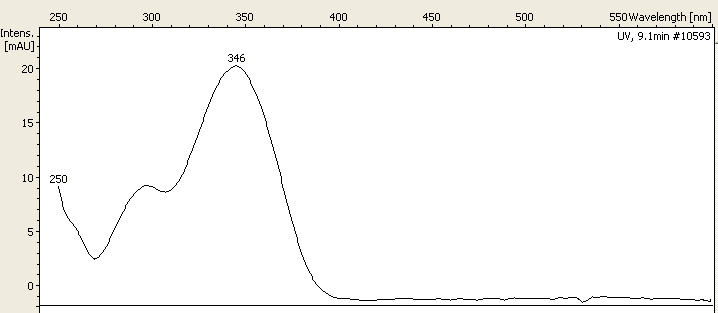
Aesculetin
Aesculetin (also known as esculetin, 6,7-dihydroxycoumarin and cichorigenin) is a derivative of coumarin. It is a natural lactone that derives from the intramolecular cyclization of a cinnamic acid derivative. It is present in chicory and in many toxic plant, toxic and medicinal plants, in form of glycosides and caffeic acid conjugates. This compound is used in some sunscreens, but there is evidence that it acts as a photosensitizer for DNA damage. The sodium salt of its methyl-derivative is used in dermatology for the treatment of varicose veins. It is a blue fluorescence compound found in plants. Aesculin, the glucoside of aesculetin, will fluorescence, fluoresce under long wave ultraviolet light (360 nanometre, nm). The hydrolysis of aesculin results in loss of this fluorescence. Aesculetin has the ability to quench the inner fluorescence of bovine serum albumin. Aesculetin can be transformed into scopoletin (7-hydroxy-6-methoxycoumarin) and isoscopoletin (6-hydroxy-7-me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Coumarins
Coumarin derivatives are derivatives of coumarin and are considered phenylpropanoids. Among the most important derivatives are the 4-hydroxycoumarins, which exhibit anticoagulant properties, a characteristic not present for coumarin itself. Some naturally occurring coumarin derivatives include umbelliferone (7-hydroxycoumarin), aesculetin (6,7-dihydroxycoumarin), herniarin (7-methoxycoumarin), psoralen and imperatorin. 4-Phenylcoumarin is the backbone of the neoflavones, a type of neoflavonoids. Coumarin pyrazole hybrids have been synthesized from hydrazones, carbazones and thiocarbazones via Vilsmeier Haack formylation reaction. Compounds derived from coumarin are also called coumarins or coumarinoids; this family includes: * brodifacoum * bromadiolone * difenacoum * auraptene * ensaculin * phenprocoumon (Marcoumar) * PSB-SB-487 * PSB-SB-1202 * scopoletin can be isolated from the bark of ''Shorea pinanga'' * warfarin (Coumadin) Coumarin is transformed into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chicory
Common chicory (''Cichorium intybus'') is a somewhat woody, perennial herbaceous plant of the family Asteraceae, usually with bright blue flowers, rarely white or pink. Native to Europe, it has been introduced to the Americas and Australia. Many varieties are cultivated for salad leaves, chicons ( blanched buds), or roots (var. ''sativum''), which are baked, ground, and used as a coffee substitute and food additive. In the 21st century, inulin, an extract from chicory root, has been used in food manufacturing as a sweetener and source of dietary fiber. Chicory is also grown as a forage crop for livestock. Description When flowering, chicory has a tough, grooved, and more or less hairy stem. It can grow to tall. The leaves are stalked, lanceolate and unlobed; they range from in length (smallest near the top) and wide. The flower heads are wide, and usually light blue or lavender; it has also rarely been described as white or pink. Of the two rows of involucral bracts, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Glucoside
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes. The name was originally given to plant products of this nature, in which the other part of the molecule was, in the greater number of cases, an aromatic aldehydic or phenolic compound (exceptions are Jinigrin and Jalapin or Scammonin). It has now been extended to include synthetic ethers, such as those obtained by acting on alcoholic glucose solutions with hydrochloric acid, and also the polysaccharoses, e.g. cane sugar, which appear to be ethers also. Although glucose is the most common sugar present in glucosides, many are known which yield rhamnose or iso-dulcite; these may be termed pentosides. Much attention has been given to the non-sugar parts (aglycone) of the molecules; the constitutions of many have been determined, and the comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aesculin
Aesculin, also called æsculin or esculin, is a coumarin glucoside that naturally occurs in the trees horse chestnut (''Aesculus hippocastanum''), California buckeye (''Aesculus californica''), and prickly box (''Bursaria spinosa''). It is also found in daphnin (the dark green resin of '' Daphne mezereum''), dandelion coffee, and olive bark. It is reported to be present in olive bark, but not in olive leaf; therefore, identification of aesculin in abundance in an olive extract indicates that the extract has been derived from olive bark. Uses Aesculin is also used in a microbiology laboratory to aid in the identification of bacterial species (especially ''Enterococci'' and Listeria). In fact, all strains of Group D Streptococci hydrolyze aesculin in 40% bile. Aesculin hydrolysis test Aesculin is incorporated into agar with ferric citrate and bile salts ( bile aesculin agar). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops. This difference in duration is a result of quantum spin effects. Fluorescence occurs when a photon from incoming radiation is absorbed by a molecule, exciting it to a higher energy level, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer wavelength and, therefore, a lower photon energy than the absorbed radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Umbelliferone
Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and ''beta''-umbelliferone, is a natural product of the coumarin family. It absorbs ultraviolet light strongly at several wavelengths. There are some indications that this chemical is antimutagenic, it is used in sunscreens. Umbelliferone has been reported to have antioxidant properties. It is a yellowish-white crystalline solid that has a slight solubility in hot water, but high solubility in ethanol. Natural occurrences and name Umbelliferone's name is from the umbelliferae family of plants, and the plant family in turn was named for their umbrella-shaped inflorescences, each called an umbel. Umbelliferone occurs in many familiar plants from the Apiaceae (Umbelliferae) family such as carrot, coriander and garden angelica, as well as in plants from other families, such as the mouse-ear hawkweed (''Hieracium pilosella'', Asteraceae) or the bigleaf hydrangea (''Hydrangea macrophylla'', Hydrangeaceae, under ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Catechol-O-methyltransferase
Catechol-''O''-methyltransferase (COMT; ) is one of several enzymes that degrade catecholamines (neurotransmitters such as dopamine, epinephrine, and norepinephrine), catecholestrogens, and various drugs and substances having a catechol structure. In humans, catechol-''O''-methyltransferase protein is encoded by the COMT gene. Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines. COMT was first discovered by the biochemist Julius Axelrod in 1957. Function Catechol-''O''-methyltransferase is involved in the inactivation of the catecholamine neurotransmitters (dopamine, epinephrine, and norepinephrine). The enzyme introduces a methyl group to the catecholamine, which is donated by S-adenosyl methionine (SAM). Any compound having ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Scopoletin
Scopoletin is a coumarin found in the root of plants in the genus '' Scopolia'' such as ''Scopolia carniolica'' and '' Scopolia japonica'', in chicory, in '' Artemisia scoparia'', in the roots and leaves of stinging nettle (''Urtica dioica''), in the passion flower, in '' Brunfelsia'', in ''Viburnum prunifolium'', in ''Solanum nigrum'', in ''Datura metel'', in '' Mallotus resinosus'', and in ''Kleinhovia hospita''. It can also be found in fenugreek, vinegar, some whiskies and in dandelion coffee. A similar coumarin is scoparone. Scopoletin is highly fluorescent when dissolved in DMSO or water and is regularly used as a fluorimetric assay for the detection of hydrogen peroxide in conjunction with horseradish peroxidase. When oxidized, its fluorescence is strongly suppressed. Chemistry Biosynthesis Like most phenylpropanoids, the biosynthetic precursor to scopoletin acid is 4-coumaroyl-CoA. Scopoletin is derived from 1,2-benzopyrones which is the core structure of coumarins for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Bovine Serum Albumin
Bovine serum albumin (BSA or "Fraction V") is a serum albumin protein derived from cows. It is often used as a protein concentration standard in lab experiments. The nickname "Fraction V" refers to albumin being the fifth fraction of the original Edwin Cohn purification methodology that made use of differential solubility characteristics of plasma proteins. By manipulating solvent concentrations, pH, salt levels, and temperature, Cohn was able to pull out successive "fractions" of blood plasma. The process was first commercialized with human albumin for medical use and later adopted for production of BSA. Properties The full-length BSA precursor polypeptide is 607 amino acids (AAs) in length. An N-terminal 18-residue signal peptide is cut off from the precursor protein upon secretion, hence the initial protein product contains 589 amino acid residues. An additional six amino acids are cleaved to yield the mature BSA protein that contains 583 amino acids. BSA has thre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nanometre
330px, Different lengths as in respect to the Molecule">molecular scale. The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm), or nanometer (American spelling), is a unit of length in the International System of Units (SI), equal to one billionth ( short scale) or one thousand million (long scale) of a meter (0.000000001 m) and to 1000 picometres. One nanometre can be expressed in scientific notation as 1 × 10−9 m and as m. History The nanometre was formerly known as the "''millimicrometre''" – or, more commonly, the "''millimicron''" for short – since it is of a micrometer. It was often denoted by the symbol ''mμ'' or, more rarely, as ''μμ'' (however, ''μμ'' should refer to a ''millionth'' of a micron). Etymology The name combines the SI prefix '' nano-'' (from the Ancient Greek , ', "dwarf") with the parent unit name ''metre'' (from Greek , ', "unit of measurement"). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |