|
Aerostatic
A subfield of fluid statics, aerostatics is the study of gases that are not in motion with respect to the coordinate system in which they are considered. The corresponding study of gases in motion is called aerodynamics. Aerostatics studies density allocation, especially in air. One of the applications of this is the barometric formula. An aerostat is a lighter than air craft, such as an airship or balloon, which uses the principles of aerostatics to float. Basic laws Treatment of the equations of gaseous behaviour at rest is generally taken, as in hydrostatics, to begin with a consideration of the general equations of momentum for fluid flow, which can be expressed as: \rho + U_i = - - + \rho g_j , where \rho is the mass density of the fluid, U_j is the instantaneous velocity, P is fluid pressure, g are the external body forces acting on the fluid, and \tau_ is the momentum transport coefficient. As the fluid's static nature mandates that U_j = 0 , and that \ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Airship
An airship or dirigible balloon is a type of aerostat or lighter-than-air aircraft that can navigate through the air under its own power. Aerostats gain their lift from a lifting gas that is less dense than the surrounding air. In early dirigibles, the lifting gas used was hydrogen, due to its high lifting capacity and ready availability. Helium gas has almost the same lifting capacity and is not flammable, unlike hydrogen, but is rare and relatively expensive. Significant amounts were first discovered in the United States and for a while helium was only available for airships in that country. Most airships built since the 1960s have used helium, though some have used hot air.A few airships after World War II used hydrogen. The first British airship to use helium was the ''Chitty Bang Bang'' of 1967. The envelope of an airship may form the gasbag, or it may contain a number of gas-filled cells. An airship also has engines, crew, and optionally also payload accommodat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerostat
An aerostat (, via French) is a lifting gas, lighter-than-air aircraft that gains its lift through the use of a buoyant gas. Aerostats include unpowered balloon (aircraft), balloons and powered airships. A balloon may be free-flying or Moored balloon, tethered. The average density of the craft is lower than the density of atmospheric air, because its main component is one or more gasbags, a lightweight skin containing a lifting gas (which could be heated air or any gas of lower density than air) to provide buoyancy, to which other components such as a gondola containing equipment or people are attached. Especially with airships, the gasbags are often protected by an outer envelope. Aerostats are so named because they use aerostatic lift which is a buoyant force that does not require movement through the surrounding air mass, resulting in VTOL ability. This contrasts with the heavy Heavier-than-air aircraft, aerodynes that primarily use aerodynamic lift (force), lift which requir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lighter Than Air
A lifting gas or lighter-than-air gas is a gas that has a density lower than normal atmospheric gases and rises above them as a result. It is required for aerostats to create buoyancy, particularly in lighter-than-air aircraft, which include free balloons, moored balloons, and airships. Only certain lighter than air gases are suitable as lifting gases. Dry air has a density of about 1.29 g/L (gram per liter) at standard conditions for temperature and pressure (STP) and an average molecular mass of 28.97 g/mol, and so lighter-than-air gases have a density lower than this. Gases used for lifting Hot air Heated atmospheric air is frequently used in recreational ballooning. According to the Ideal gas law, an amount of gas (and also a mixture of gases such as air) expands as it is heated. As a result, a certain volume of gas has a lower density as the temperature is higher. The average temperature of air in a hot air balloon is about . Hydrogen Hydrogen, being the l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluid Statics
Fluid statics or hydrostatics is the branch of fluid mechanics that studies the condition of the equilibrium of a floating body and submerged body "fluids at hydrostatic equilibrium and the pressure in a fluid, or exerted by a fluid, on an immersed body". It encompasses the study of the conditions under which fluids are at rest in stable equilibrium as opposed to fluid dynamics, the study of fluids in motion. Hydrostatics is a subcategory of fluid statics, which is the study of all fluids, both compressible or incompressible, at rest. Hydrostatics is fundamental to hydraulics, the engineering of equipment for storing, transporting and using fluids. It is also relevant to geophysics and astrophysics (for example, in understanding plate tectonics and the anomalies of the Earth's gravitational field), to meteorology, to medicine (in the context of blood pressure), and many other fields. Hydrostatics offers physical explanations for many phenomena of everyday life, such as why a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosphere is the outer region of a star, which includes the layers above the opaque photosphere; stars of low temperature might have outer atmospheres containing compound molecules. The atmosphere of Earth is composed of nitrogen (78%), oxygen (21%), argon (0.9%), carbon dioxide (0.04%) and trace gases. Most organisms use oxygen for respiration; lightning and bacteria perform nitrogen fixation to produce ammonia that is used to make nucleotides and amino acids; plants, algae, and cyanobacteria use carbon dioxide for photosynthesis. The layered composition of the atmosphere minimises the harmful effects of sunlight, ultraviolet radiation, the solar wind, and cosmic rays to protect organisms from genetic damage. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aeronautics
Aeronautics is the science or art involved with the study, design, and manufacturing of air flight–capable machines, and the techniques of operating aircraft and rockets within the atmosphere. The British Royal Aeronautical Society identifies the aspects of "aeronautical Art, Science and Engineering" and "The profession of Aeronautics (which expression includes Astronautics)." While the term originally referred solely to ''operating'' the aircraft, it has since been expanded to include technology, business, and other aspects related to aircraft. The term "aviation" is sometimes used interchangeably with aeronautics, although "aeronautics" includes lighter-than-air craft such as airships, and includes ballistic vehicles while "aviation" technically does not. A significant part of aeronautical science is a branch of dynamics called aerodynamics, which deals with the motion of air and the way that it interacts with objects in motion, such as an aircraft. History Early i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
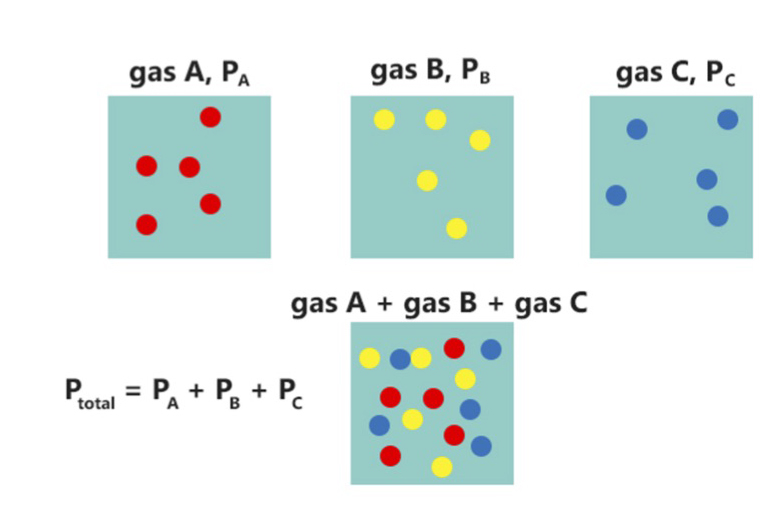
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture ( Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetic Theory Of Gases
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to: * Kinetic theory, describing a gas as particles in random motion * Kinetic energy In physics, the kinetic energy of an object is the energy that it possesses due to its motion. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its a ..., the energy of an object that it possesses due to its motion Art and entertainment * Kinetic art, a form of art involving mechanical and/or random movement, including optical illusions. * ''Kinetic'', the 13th episode of the first season of the TV series ''Smallville'' * ''Kinetic'' (comics), a comic by Allan Heinberg and Kelley Pucklett * "Kinetic" (song), a song by Radiohead Companies * Kinetic Engineering Limited, Indian automotive manufacturer * Kinetic Group, Australian-based public transport company Technology * "Kinetic", Seiko's trademark for its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soil Gas
Soil gases (soil atmosphere) are the gases found in the air space between soil components. The spaces between the solid soil particles, if they do not contain water, are filled with air. The primary soil gases are nitrogen, carbon dioxide and oxygen. Oxygen is critical because it allows for respiration of both plant roots and soil organisms. Other natural soil gases include nitric oxide, nitrous oxide, methane, and ammonia. Some environmental contaminants below ground produce gas which diffuses through the soil such as from landfill wastes, mining activities, and contamination by petroleum hydrocarbons which produce volatile organic compounds. Gases fill soil pores in the soil structure as water drains or is removed from a soil pore by evaporation or root absorption. The network of pores within the soil aerates, or ventilates, the soil. This aeration network becomes blocked when water enters soil pores. Not only are both soil air and soil water very dynamic parts of soil, but bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Gas Law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: pV = nRT where p, V and T are the pressure, volume and temperature; n is the amount of substance; and R is the ideal gas constant. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856 and Rudolf Clausius in 1857. Equation The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atmospheric Pressure
Atmospheric pressure, also known as barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1013.25 millibars, 760 mm Hg, 29.9212 inchesHg, or 14.696 psi.International Civil Aviation Organization. ''Manual of the ICAO Standard Atmosphere'', Doc 7488-CD, Third Edition, 1993. . The atm unit is roughly equivalent to the mean sea-level atmospheric pressure on Earth; that is, the Earth's atmospheric pressure at sea level is approximately 1 atm. In most circumstances, atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point. As elevation increases, there is less overlying atmospheric mass, so atmospheric pressure decreases with increasing elevation. Because the atmosphere is thin relative to the Earth's radius—especially the dense atmospheric layer at low altitudes—the E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |