|
Volhard–Erdmann Cyclization
The Volhard–Erdmann cyclization is an organic synthesis of alkyl and aryl thiophenes by cyclization of disodium succinate or other 1,4-difunctional compounds (γ-oxo acids, 1,4-diketones, chloroacetyl-substituted esters) with phosphorus heptasulfide. The reaction is named after Jacob Volhard and Hugo Erdmann. An example is the synthesis of 3-methylthiophene starting from itaconic acid Itaconic acid, or methylidenesuccinic acid, is an organic compound. This dicarboxylic acid is a white solid that is soluble in water, ethanol, and acetone. Historically, itaconic acid was obtained by the distillation of citric acid, but currently ...: : References {{DEFAULTSORT:Volhard-Erdmann cyclization Sulfur heterocycle forming reactions Name reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: '' total synthesis'', '' semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
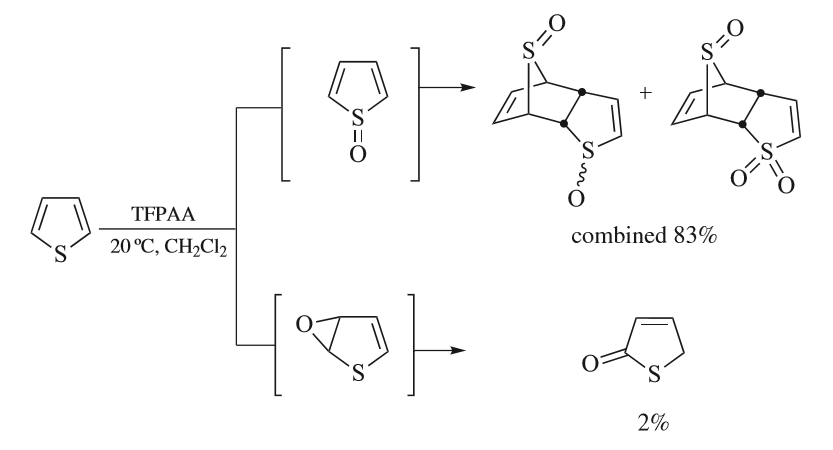
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinate
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin ''succinum'', meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological roles as a metabolic intermediate being converted into fumarate by the enzyme succinate dehydrogenase in complex 2 of the electron transport chain which is involved in making ATP, and as a signaling molecule reflecting the cellular metabolic state. It is marketed as food additive E363. Succinate is generated in mitochondria via the tricarboxylic acid cycle (TCA). Succinate can exit the mitochondrial matrix and function in the cytoplasm as well as the extracellular space, changing gene expression patterns, modulating epigenetic landscape or demonstrating hormone-like signaling. As such, succinate links cellular metabolism, especially ATP formation, to the regulation of cellular function. Dysregulation of succinate synthesis, and therefore A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Sulfide
Phosphorus sulfides comprise a family of inorganic compounds containing only phosphorus and sulfur. These compounds have the formula with ''n'' ≤ 10. Two are of commercial significance, phosphorus pentasulfide (), which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide (), used in the production of "strike anywhere matches". There are several other phosphorus sulfides in addition to and . Six of these phosphorus sulfides exist as isomers: . These isomers are distinguished by Greek letter prefixes. The prefix is based on the order of the discovery of the isomers, not their structure. All known molecular phosphorus sulfides contain a tetrahedral array of four phosphorus atoms. is also known but is unstable above −30 °C. Preparation The main method for preparing these compounds is thermolysis of mixtures of phosphorus and sulfur. The product distributions can be analyzed by 31P-NMR spectroscopy. More selective ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jacob Volhard
Jacob Volhard (4 June 1834 – 14 January 1910) was the German chemist who discovered, together with his student Hugo Erdmann, the Volhard–Erdmann cyclization reaction. He was also responsible for the improvement of the Hell–Volhard–Zelinsky halogenation. From 1852 to 1855 he studied chemistry at the University of Giessen, and afterwards, furthered his education at the University of Heidelberg. For two years he worked as an assistant under Justus von Liebig at the University of Munich, and in 1860/61 studied with August Wilhelm von Hofmann in London. In 1863 he obtained his habilitation at Munich, where he subsequently became an associate professor. In the meantime, he worked in the Institute of Plant Physiology at the Bavarian Academy of Sciences and Humanities, Bavarian Academy of Sciences (1865–76). In 1879 he was named a professor of organic chemistry at the University of Erlangen, then in 1882 relocated to the University of Halle, where he served as a professor up ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hugo Erdmann
Hugo Wilhelm Traugott Erdmann (8 May 1862 – 25 June 1910) was the German chemist who discovered, together with his doctoral advisor Jacob Volhard, the Volhard-Erdmann cyclization. In 1898 he was the first who coined the term ''noble gas'' (the original noun is in German). Erdmann invented the name Thiozone in 1908, hypothesizing that S3 made up a large proportion of liquid sulfur. In collaboration with Rudolph Fittig, Erdmann found that dehydration of γ-phenyl structural analog of isocrotonic acid produced α-naphthol, an observation that provided evidence in understanding the nature of naphthalene Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromat .... Bibliography Books written by Erdmann: # See also * German inventors and discoverers References * * Notes 1862 b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Itaconic Acid
Itaconic acid, or methylidenesuccinic acid, is an organic compound. This dicarboxylic acid is a white solid that is soluble in water, ethanol, and acetone. Historically, itaconic acid was obtained by the distillation of citric acid, but currently it is produced by fermentation. The name ''itaconic acid'' was devised as an anagram of aconitic acid, another derivative of citric acid. Production Since the 1960s, it is produced industrially by the fermentation of carbohydrates such as glucose or molasses using fungi such as ''Aspergillus itaconicus'' or ''Aspergillus terreus''. For ''A. terreus'' the itaconate pathway is mostly elucidated. The generally accepted route for itaconate is via glycolysis, tricarboxylic acid cycle, and a decarboxylation of ''cis''-aconitate to itaconate via ''cis''-aconitate-decarboxylase. The smut fungus ''Ustilago maydis'' uses an alternative route. ''Cis''-aconitate is converted to the thermodynamically favoured ''trans''-aconitate via aconitate-� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemische Berichte
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with ''Recueil des Travaux Chimiques des Pays-Bas'' to form ''Chemische Berichte/Recueil'' in 1997. ''Chemische Berichte/Recueil'' was then merged with other European journals in 1998 to form ''European Journal of Inorganic Chemistry''. History Founded in 1868 as ''Berichte der Deutschen Chemischen Gesellschaft'' (, CODEN BDCGAS), it operated under this title until 1928 (Vol. 61). The journal was then split into: * ''Berichte der Deutschen Chemischen Gesellschaft, A: Vereins-Nachrichten'' (, CODEN BDCAAS), and * ''Berichte der Deutschen Chemischen Gesellschaft, B: Abhandlungen'' (, CODEN BDCBAD). Vol. 78 and 79 (1945–1946) were omitted and not published due to World War II. The journal was renamed ''Chemische Berichte'' (, CODEN CHBEAM) in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Heterocycle Forming Reactions
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the production of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |