|
Vinyl Fluoride
Vinyl fluoride is an organic halide with the chemical formula C2H3F. It is a colorless gas with a faint ether-like odor. It is used as the monomeric precursor to the fluoropolymer polyvinylfluoride. Production It was first prepared in 1901 by Frédéric Swarts, the Belgian chemist who was the first to prepare chlorofluorocarbons in 1892. Swarts used the reaction of zinc with 1,1-difluoro-2-bromoethane. It is produced industrially by two routes, one being the mercury-catalyzed reaction of acetylene and hydrogen fluoride:Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick “Fluorine Compounds, Organic” Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. :HC≡CH + HF → CH2=CHF It is also prepared from 1,1-chlorofluoroethane: :CH3CHClF → CH2=CHF + HCl Safety Vinyl fluoride is classified as an IARC Group 2A carcinogen (likely to cause cancer in humans). Additional data Its critica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Halide
Halocarbon compounds are Chemical compound, chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides. Many synthetic organic compounds such as plastic polymers, and a few natural ones, contain halogen atoms; they are known as ''halogenated'' compounds or ''organohalogens''. Organochlorides are the most common industrially used organohalides, although the other organohalides are used commonly in organic synthesis. Except for extremely rare cases, organohalides are not produced biologically, but many pharmaceuticals are organohalides. Notably, many pharmaceuticals such as Fluoxetine, Prozac have trifluoromethyl groups. For information on inorganic halide chemistry, see halide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible Signs and symptoms of cancer, signs and symptoms include a lump, abnormal bleeding, prolonged cough, unexplained weight loss, and a change in defecation, bowel movements. While these symptoms may indicate cancer, they can also have other causes. List of cancer types, Over 100 types of cancers affect humans. Tobacco use is the cause of about 22% of cancer deaths. Another 10% are due to obesity, poor Diet (nutrition), diet, sedentary lifestyle, lack of physical activity or Alcohol abuse, excessive alcohol consumption. Other factors include certain infections, exposure to ionizing radiation, and environmental pollutants. infectious causes of cancer, Infection with specific viruses, bacteria and parasites is an environmental factor cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IARC Group 2A Carcinogens
IARC group 2A agents are substances and exposure circumstances that have been classified as probable carcinogens by the International Agency for Research on Cancer (IARC). This designation is applied when there is limited evidence of carcinogenicity in humans, as well as ''sufficient evidence'' of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is ''inadequate evidence'' of carcinogenicity in humans along with ''sufficient evidence'' of carcinogenicity in experimental animals and ''strong evidence'' that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of ''limited evidence'' of carcinogenicity in humans. This list is focusing on the hazard linked to the agents. This means that the carcinogenic agents are capable of causing cancer, but this does not take their risk into account, which is the probability of causing a can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refrigerants
A refrigerant is a working fluid used in the cooling, heating, or reverse cooling/heating cycles of air conditioning systems and heat pumps, where they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated because of their toxicity and flammability, as well as the contribution of CFC and HCFC refrigerants to ozone depletion and the contribution of HFC refrigerants to climate change. Refrigerants are used in a direct expansion (DX) circulating system to transfer energy from one environment to another, typically from inside a building to outside or vice versa. These can be air conditioner cooling only systems, cooling & heating reverse DX systems, or heat pump and heating only DX cycles. Refrigerants are controlled substances that are classified by several international safety regulations and, depending on their classification, may only be handled by qualified engineers due to extreme pressure, temperature, flammability, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Bromide
Vinyl bromide is the organobromine compound with the formula . Classified as a vinyl halide, it is a colorless gas at room temperature. It is used as a reagent and a comonomer. Synthesis, reactions, and applications It is produced from ethylene dibromide: : is mainly consumted as a comonomer to confer fire retardant properties to acrylate polymers. Vinyl bromide reacts with magnesium to give the corresponding Grignard reagent . Safety precautions Vinyl bromide is listed in List of IARC Group 2A carcinogens as a suspected human carcinogen. See also * Vinyl chloride * Allyl bromide * Bromoethane Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr (which is also used as an abbreviation for ethidium bromide). This volatile compound has an ether-like odor. Prepara ... References External links * *MSDS at Oxford University [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Chloride
Vinyl chloride is an organochloride with the formula H2C =CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. It is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). Vinyl chloride is a colourless flammable gas that has a sweet odor and is carcinogenic. Vinyl chloride monomer is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States remains the largest vinyl chloride manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of vinyl chloride. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common contaminant found near landfills. Before the 197 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Of Vaporization
In thermodynamics, the enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to Phase transition, transform a quantity of that substance into a gas. The enthalpy of vaporization is a function of the pressure and temperature at which the transformation (vaporization or evaporation) takes place. The enthalpy of vaporization is often quoted for the normal boiling point, normal boiling temperature of the substance. Although tabulated values are usually corrected to 298 Kelvin, K, that correction is often smaller than the Significant figures, uncertainty in the measured value. The heat of vaporization is temperature-dependent, though a constant heat of vaporization can be assumed for small temperature ranges and for reduced temperature . The heat of vaporization diminishes with increasing temperature and it vanishes completely at a certain point cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Dipole Moment
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways: * An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system is a pair of charges of equal magnitude but opposite sign separated by some typically small distance. (A permanent electric dipole is called an electret.) * A magnetic dipole is the closed circulation of an electric current system. A simple example is a single loop of wire with constant current through it. A bar magnet is an example of a magnet with a permanent magnetic dipole moment. Dipoles, whether electric or magnetic, can be characterized by their dipole moment, a vector quantity. For the simple electric dipole, the electric dipole moment points from the negative charge towards the positive charge, and has a magnitude equal to the strength of each charge times the separation between the charges. (To be precise: for the definit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of IARC Group 2A Carcinogens
IARC group 2A agents are substances and exposure circumstances that have been classified as probable carcinogens by the International Agency for Research on Cancer (IARC). This designation is applied when there is limited evidence of carcinogenicity in humans, as well as ''sufficient evidence'' of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is ''inadequate evidence'' of carcinogenicity in humans along with ''sufficient evidence'' of carcinogenicity in experimental animals and ''strong evidence'' that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of ''limited evidence'' of carcinogenicity in humans. This list is focusing on the hazard linked to the agents. This means that the carcinogenic agents are capable of causing cancer, but this does not take their risk into account, which is the probability of causing a can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
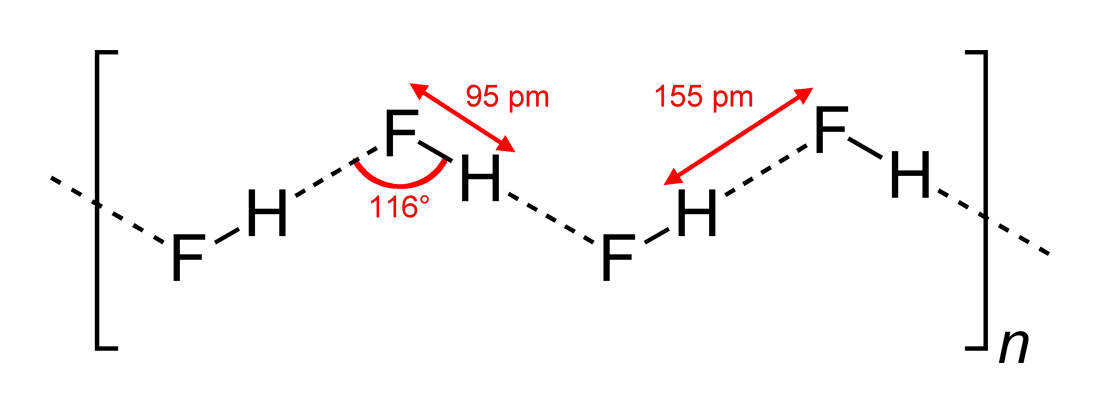
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |