|
Vaccine Trial
A vaccine trial is a clinical trial that aims at establishing the safety and efficacy of a vaccine prior to it being licensed. A vaccine candidate drug is first identified through preclinical evaluations that could involve high throughput screening and selecting the proper antigen to invoke an immune response. Some vaccine trials may take months or years to complete, depending on the time required for the subjects to react to the vaccine and develop the required antibodies. Preclinical stage Preclinical development stages are necessary to determine the immunogenicity potential and safety profile for a vaccine candidate. This is also the stage in which the drug candidate may be first tested in laboratory animals prior to moving to the Phase I trials. Vaccines such as the oral polio vaccine have been first tested for adverse effects and immunogenicity in monkeys as well as non-human primates and lab mice. Scientific advances since the 1980s have helped to use transgenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covid Vaccine Clinical Trial, Padjajaran University (cropped)
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by the coronavirus SARS-CoV-2. In January 2020, the disease spread worldwide, resulting in the COVID-19 pandemic. The symptoms of COVID‑19 can vary but often include fever, fatigue, cough, breathing difficulties, loss of smell, and loss of taste. Symptoms may begin one to fourteen days after exposure to the virus. At least a third of people who are infected do not develop noticeable symptoms. Of those who develop symptoms noticeable enough to be classified as patients, most (81%) develop mild to moderate symptoms (up to mild pneumonia), while 14% develop severe symptoms (dyspnea, hypoxia, or more than 50% lung involvement on imaging), and 5% develop critical symptoms (respiratory failure, shock (circulatory), shock, or organ dysfunction, multiorgan dysfunction). Older people have a higher risk of developing severe symptoms. Some complications result in death. Some people continue to experience a range of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo-controlled Studies
Placebo-controlled studies are a way of testing a medical therapy in which, in addition to a group of subjects that receives the treatment to be evaluated, a separate control group receives a sham "placebo" treatment which is specifically designed to have no real effect. Placebos are most commonly used in blinded trials, where subjects do not know whether they are receiving real or placebo treatment. Often, there is also a further "natural history" group that does not receive any treatment at all. The purpose of the placebo group is to account for the placebo effect, that is, effects from treatment that do not depend on the treatment itself. Such factors include knowing one is receiving a treatment, attention from health care professionals, and the expectations of a treatment's effectiveness by those running the research study. Without a placebo group to compare against, it is not possible to know whether the treatment itself had any effect. Patients frequently show improvement ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerivastatin
Cerivastatin ( INN, brand names: Baycol, Lipobay) is a synthetic member of the class of statins used to lower cholesterol and prevent cardiovascular disease. It was marketed by the pharmaceutical company Bayer A.G. in the late 1990s, competing with Pfizer's highly successful atorvastatin (Lipitor). Cerivastatin was voluntarily withdrawn from the market worldwide in 2001, due to reports of fatal rhabdomyolysis. During postmarketing surveillance, 52 deaths were reported in patients using cerivastatin, mainly from rhabdomyolysis and its resultant kidney failure. Risks were higher in patients using fibrates, mainly gemfibrozil (Lopid), and in patients using the highest (0.8 mg/day) dose of cerivastatin. Bayer A.G. added a contraindication for the concomitant use of cerivastatin and gemfibrozil to the package 18 months after the drug interaction was found. The frequency of deadly cases of rhabdomyolysis with cerivastatin was 16 to 80 times higher than with other statins. Anothe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
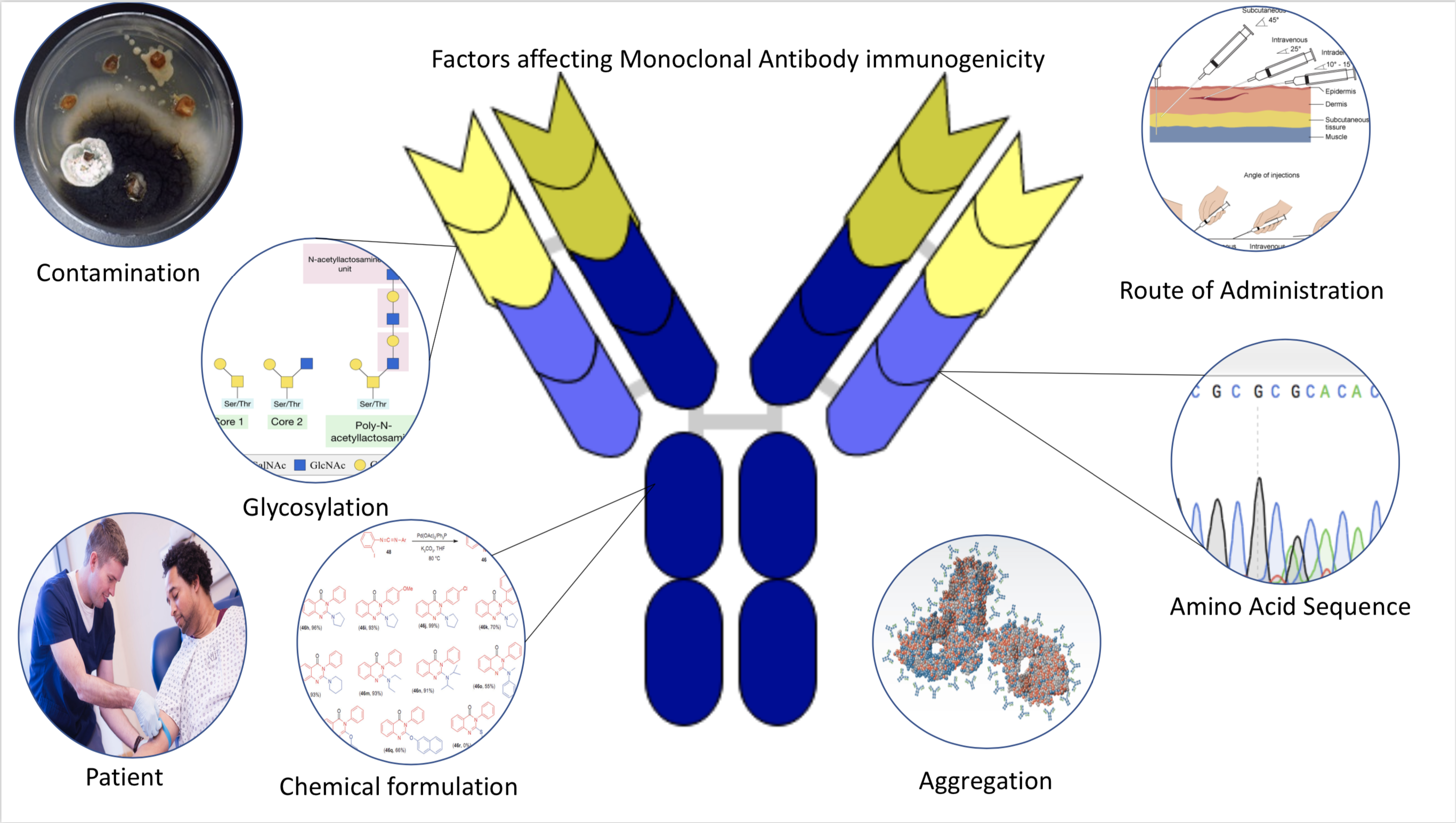
Immunogenicity
Immunogenicity is the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal. It may be wanted or unwanted: * Wanted immunogenicity typically relates to vaccines, where the injection of an antigen (the vaccine) provokes an immune response against the pathogen, protecting the organism from future exposure. Immunogenicity is a central aspect of vaccine development. * Unwanted immunogenicity is an immune response by an organism against a therapeutic antigen. This reaction leads to production of anti-drug-antibodies (ADAs), inactivating the therapeutic effects of the treatment and potentially inducing adverse effects. A challenge in biotherapy is predicting the immunogenic potential of novel protein therapeutics. For example, immunogenicity data from high-income countries are not always transferable to low-income and middle-income countries. Another challenge is considering how the immunogenicity of vaccines changes wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunity (medical)
In biology, immunity is the state of being insusceptible or resistant to a noxious agent or process, especially a pathogen or infectious disease. Immunity may occur naturally or be produced by prior exposure or immunization. Innate and adaptive The immune system has Innate immune system, innate and Adaptive immune system, adaptive components. Innate immunity is present in all metazoans, immune responses: inflammation, inflammatory responses and phagocytosis. The adaptive component, on the other hand, involves more advanced lymphocyte, lymphatic cells that can distinguish between specific "non-self" substances in the presence of "self". The reaction to foreign substances is etymologically described as inflammation while the non-reaction to self substances is described as immunity. The two components of the immune system create a dynamic biological environment where "health" can be seen as a physical state where the self is immunologically spared, and what is foreign is inflammat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adverse Effect (medicine)
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. The term complication is similar to adverse effect, but the latter is typically used in pharmacological contexts, or when the negative effect is expected or common. If the negative effect results from an unsuitable or incorrect dosage or procedure, this is called a medical error and not an adverse effect. Adverse effects are sometimes referred to as " iatrogenic" because they are generated by a physician/treatment. Some adverse effects occur only when starting, increasing or discontinuing a treatment. Using a drug or other medical intervention which is contraindicated may increase the risk of adverse effects. Adverse effects may cause complications of a disease or procedure and negatively affect its prognosis. They may also lead to non-compliance with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistically Significant
In statistical hypothesis testing, a result has statistical significance when a result at least as "extreme" would be very infrequent if the null hypothesis were true. More precisely, a study's defined significance level, denoted by \alpha, is the probability of the study rejecting the null hypothesis, given that the null hypothesis is true; and the ''p''-value of a result, ''p'', is the probability of obtaining a result at least as extreme, given that the null hypothesis is true. The result is said to be ''statistically significant'', by the standards of the study, when p \le \alpha. The significance level for a study is chosen before data collection, and is typically set to 5% or much lower—depending on the field of study. In any experiment or observation that involves drawing a sample from a population, there is always the possibility that an observed effect would have occurred due to sampling error alone. But if the ''p''-value of an observed effect is less than (or equal to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
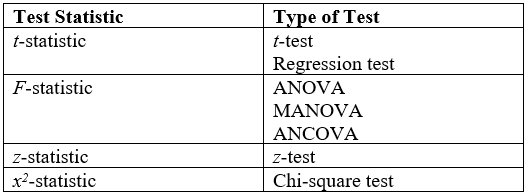
Statistical Hypothesis Testing
A statistical hypothesis test is a method of statistical inference used to decide whether the data provide sufficient evidence to reject a particular hypothesis. A statistical hypothesis test typically involves a calculation of a test statistic. Then a decision is made, either by comparing the test statistic to a Critical value (statistics), critical value or equivalently by evaluating a p-value, ''p''-value computed from the test statistic. Roughly 100 list of statistical tests, specialized statistical tests are in use and noteworthy. History While hypothesis testing was popularized early in the 20th century, early forms were used in the 1700s. The first use is credited to John Arbuthnot (1710), followed by Pierre-Simon Laplace (1770s), in analyzing the human sex ratio at birth; see . Choice of null hypothesis Paul Meehl has argued that the epistemological importance of the choice of null hypothesis has gone largely unacknowledged. When the null hypothesis is predicted by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protocol (natural Sciences)
In natural and social science research, a protocol is most commonly a predefined procedural method in the design and implementation of an experiment. Protocols are written whenever it is desirable to standardize a laboratory method to ensure successful replication of results by others in the same laboratory or by other laboratories. Additionally, and by extension, protocols have the advantage of facilitating the assessment of experimental results through peer review. In addition to detailed procedures, equipment, and instruments, protocols will also contain study objectives, reasoning for experimental design, reasoning for chosen sample sizes, safety precautions, and how results were calculated and reported, including statistical analysis and any rules for predefining and documenting excluded data to avoid bias. Similarly, a protocol may refer to the procedural methods of health organizations, commercial laboratories, manufacturing plants, etc. to ensure their activities (e.g., ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, including those that cause disease. Each individual antibody recognizes one or more specific antigens, and antigens of virtually any size and chemical composition can be recognized. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each of the branching chains comprising the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" the antigen (or a microbe or an infected cell bearing such an antigen) for attack by cells of the immune system, or can neutralize it directly (for example, by blocking a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunogenicity
Immunogenicity is the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal. It may be wanted or unwanted: * Wanted immunogenicity typically relates to vaccines, where the injection of an antigen (the vaccine) provokes an immune response against the pathogen, protecting the organism from future exposure. Immunogenicity is a central aspect of vaccine development. * Unwanted immunogenicity is an immune response by an organism against a therapeutic antigen. This reaction leads to production of anti-drug-antibodies (ADAs), inactivating the therapeutic effects of the treatment and potentially inducing adverse effects. A challenge in biotherapy is predicting the immunogenic potential of novel protein therapeutics. For example, immunogenicity data from high-income countries are not always transferable to low-income and middle-income countries. Another challenge is considering how the immunogenicity of vaccines changes wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |