|
Urey–Bigeleisen–Mayer Equation
In stable isotope geochemistry, the Urey–Bigeleisen–Mayer equation, also known as the Bigeleisen–Mayer equation or the Urey model, is a model describing the approximate equilibrium isotope fractionation in an isotope exchange reaction. While the equation itself can be written in numerous forms, it is generally presented as a ratio of partition functions of the isotopic molecules involved in a given reaction. The Urey–Bigeleisen–Mayer equation is widely applied in the fields of quantum chemistry and geochemistry and is often modified or paired with other quantum chemical modelling methods (such as density functional theory) to improve accuracy and precision and reduce the computational cost of calculations. The equation was first introduced by Harold Urey and, independently, by Jacob Bigeleisen and Maria Goeppert Mayer in 1947. Description Since its original descriptions, the Urey–Bigeleisen–Mayer equation has taken many forms. Given an isotopic exchange reaction A+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Isotope Geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope-ratio mass spectrometry, and can reveal information about the ages and origins of rock, air or water bodies, or processes of mixing between them. Stable isotope geochemistry is largely concerned with isotopic variations arising from mass-dependent isotope fractionation, whereas radiogenic isotope geochemistry is concerned with the products of natural radioactivity. Stable isotope geochemistry For most stable isotopes, the magnitude of fractionation from kinetic and equilibrium fractionation is very small; for this reason, enrichments are typically reported in "per mil" (‰, parts per thousand). These enrichments (δ) represent the ratio of heavy isotope to light isotope in the sample over the ratio of a standard. That is, :\delta \ce = \left( \frac -1 \right) \times 1000 ‰ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Vibration
A molecular vibration is a Periodic function, periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The infrared spectroscopy correlation table, typical vibrational frequencies range from less than 1013 hertz, Hz to approximately 1014 Hz, corresponding to wavenumbers of approximately 300 to 3000 cm−1 and wavelengths of approximately 30 to 3 μm. Vibrations of polyatomic molecules are described in terms of normal modes, which are independent of each other, but each normal mode involves simultaneous vibrations of parts of the molecule. In general, a non-linear molecule with ''N'' atoms has vibrational mode, normal modes of vibration, but a ''linear'' molecule has modes, because rotation about the molecular axis cannot be observed. A diatomic molecule has one normal mode of vibration, since it can only stretch or compress the single bond. A molecular vibration is excited when the mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Columbia University
Columbia University in the City of New York, commonly referred to as Columbia University, is a Private university, private Ivy League research university in New York City. Established in 1754 as King's College on the grounds of Trinity Church (Manhattan), Trinity Church in Manhattan, it is the oldest institution of higher education in New York (state), New York and the fifth-First university in the United States, oldest in the United States. Columbia was established as a Colonial colleges, colonial college by royal charter under George II of Great Britain. It was renamed Columbia College (New York), Columbia College in 1784 following the American Revolution, and in 1787 was placed under Trustees of Columbia University in the City of New York, a private board of trustees headed by former students Alexander Hamilton and John Jay. In 1896, the campus was moved to its current location in Morningside Heights and renamed Columbia University. Columbia is organized into twenty schoo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heavy Water
Heavy water (deuterium oxide, , ) is a form of water (molecule), water in which hydrogen atoms are all deuterium ( or D, also known as ''heavy hydrogen'') rather than the common hydrogen-1 isotope (, also called ''protium'') that makes up most of the hydrogen in normal water. The presence of the heavier isotope gives the water different nuclear properties, and the increase in mass gives it slightly different physical and chemical properties when compared to normal water. Deuterium is a heavy Isotopes of hydrogen, hydrogen isotope. Heavy water contains deuterium atoms and is used in nuclear reactors. Semiheavy water (HDO) is more common than pure heavy water, while heavy-oxygen water is denser but lacks unique properties. Tritiated water is radioactive due to tritium content. Heavy water has different physical properties from regular water, such as being 10.6% denser and having a higher melting point. Heavy water is less Dissociation (chemistry), dissociated at a given temperatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more common H has no neutrons. The name ''deuterium'' comes from Greek '' deuteros'', meaning "second". American chemist Harold Urey discovered deuterium in 1931. Urey and others produced samples of heavy water in which the H had been highly concentrated. The discovery of deuterium won Urey a Nobel Prize in 1934. Nearly all deuterium found in nature was synthesized in the Big Bang 13.8 billion years ago, forming the primordial ratio of H to H (~26 deuterium nuclei per 10 hydrogen nuclei). Deuterium is subsequently produced by the slow stellar proton–proton chain, but rapidly destroyed by exothermic fusion reactions. The deuterium–deuterium reaction has the second-lowest energy threshold, and is the most astrophysically acce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus-32
Phosphorus-32 (32P) is a radioactive isotope of phosphorus. The nucleus of phosphorus-32 contains 15 protons and 17 neutrons, one more neutron than the most common isotope of phosphorus, phosphorus-31. Phosphorus-32 only exists in small quantities on Earth as it has a short half-life of 14 days and so decays rapidly. Phosphorus is found in many organic molecules, and so, phosphorus-32 has many applications in medicine, biochemistry, and molecular biology where it can be used to trace phosphorylated molecules (for example, in elucidating metabolic pathways) and radioactively label DNA and RNA. Decay Phosphorus-32 has a short half-life of 14.268 days and decays into sulfur-32 by beta decay as shown in this nuclear equation: : 1.709 MeV of energy is released from this decay. The kinetic energy of the electron varies with an average of approximately 0.5 MeV and the remainder of the energy is carried by the nearly undetectable electron antineutrino. In comparison to oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur-35
Sulfur (16S) has 23 known isotopes with mass numbers ranging from 27 to 49, four of which are stable: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%). The preponderance of sulfur-32 is explained by its production from carbon-12 plus successive fusion capture of five helium-4 nuclei, in the so-called alpha process of exploding type II supernovas (see silicon burning). Other than 35S, the radioactive isotopes of sulfur are all comparatively short-lived. 35S is formed from cosmic ray spallation of 40 Ar in the atmosphere. It has a half-life of 87 days. The next longest-lived radioisotope is sulfur-38, with a half-life of 170 minutes. Isotopes lighter than 32S mostly decay to isotopes of phosphorus or silicon, while 35S and heavier radioisotopes decay to isotopes of chlorine. The beams of several radioactive isotopes (such as those of 44S) have been studied theoretically within the framework of the synthesis of superheavy elements, especially those ones in the vicinity of is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic matter is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (C), which makes up 99% of all carbon on Earth; carbon-13 (C), which makes up 1%; and carbon-14 (C), which occurs in trace amounts, making up about 1-1.5 atoms per 10 atoms of carbon in the atmosphere. C and C are both stable; C is unstable, with half-life years. Carbon-14 has a specific activity of 62.4 mCi/mmol (2.31 GBq/mmol), or 164.9 GBq/g. Carbon-14 decay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manhattan Project
The Manhattan Project was a research and development program undertaken during World War II to produce the first nuclear weapons. It was led by the United States in collaboration with the United Kingdom and Canada. From 1942 to 1946, the project was directed by Major General Leslie Groves of the United States Army Corps of Engineers, U.S. Army Corps of Engineers. Nuclear physicist J. Robert Oppenheimer was the director of the Los Alamos Laboratory that designed the bombs. The Army program was designated the Manhattan District, as its first headquarters were in Manhattan; the name gradually superseded the official codename, Development of Substitute Materials, for the entire project. The project absorbed its earlier British counterpart, Tube Alloys, and subsumed the program from the American civilian Office of Scientific Research and Development. The Manhattan Project employed nearly 130,000 people at its peak and cost nearly US$2 billion (equivalent to about $ b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Path Integral Formulation
The path integral formulation is a description in quantum mechanics that generalizes the stationary action principle of classical mechanics. It replaces the classical notion of a single, unique classical trajectory for a system with a sum, or functional integral, over an infinity of quantum-mechanically possible trajectories to compute a quantum amplitude. This formulation has proven crucial to the subsequent development of theoretical physics, because manifest Lorentz covariance (time and space components of quantities enter equations in the same way) is easier to achieve than in the operator formalism of canonical quantization. Unlike previous methods, the path integral allows one to easily change coordinates between very different canonical descriptions of the same quantum system. Another advantage is that it is in practice easier to guess the correct form of the Lagrangian of a theory, which naturally enters the path integrals (for interactions of a certain type, these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
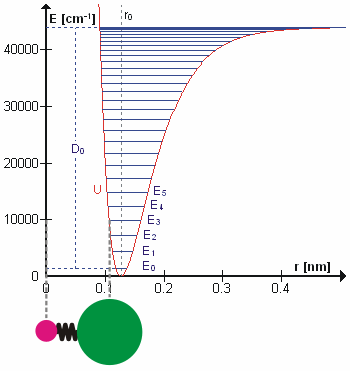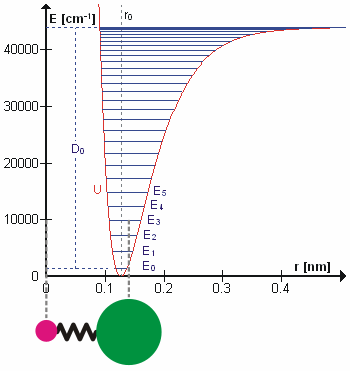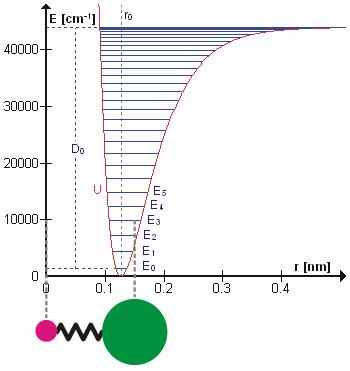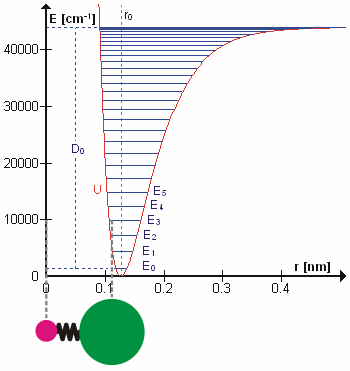
Anharmonicity
In classical mechanics, anharmonicity is the deviation of a system from being a harmonic oscillator. An oscillator that is not oscillating in harmonic motion is known as an anharmonic oscillator where the system can be approximated to a harmonic oscillator and the anharmonicity can be calculated using perturbation theory. If the anharmonicity is large, then other numerical techniques have to be used. In reality all oscillating systems are anharmonic, but most approximate the harmonic oscillator the smaller the amplitude of the oscillation is. As a result, oscillations with frequencies 2\omega and 3\omega etc., where \omega is the fundamental frequency of the oscillator, appear. Furthermore, the frequency \omega deviates from the frequency \omega_0 of the harmonic oscillations. See also intermodulation and combination tones. As a first approximation, the frequency shift \Delta \omega=\omega-\omega_0 is proportional to the square of the oscillation amplitude A: :\Delta \omeg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |