|
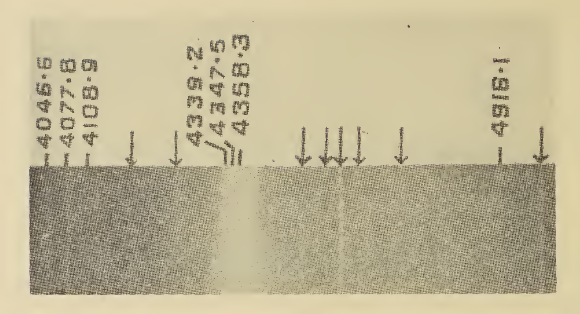
Trisulfur
The molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. It comprises about 10% of vaporised sulfur at and . It has been observed at cryogenic temperatures as a solid. Under ordinary conditions it converts to cyclooctasulfur. : Structure and bonding In terms of structure and bonding and ozone () are similar. Both adopt bent structures and are diamagnetic. Although represented with S=S double bonds, the bonding situation is more complex. The S–S distances are equivalent and are , and with an angle at the central atom of . However, cyclic , where the sulfur atoms are arranged in an equilateral triangle with three single bonds (similar to cyclic ozone and cyclopropane), is calculated to be lower in energy than the bent structure experimentally observed. A similar structure has been predicted for ozone, but has not been observed. The name thiozone was invented by Hugo Erdmann in 1908 who hypothesized that compr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfur Monoxide
Disulfur monoxide or sulfur suboxide is an inorganic compound with the formula S2O, one of the lower sulfur oxides. It is a colourless gas and condenses to give a roughly dark red coloured solid that is unstable at room temperature. occurs rarely in natural atmospheres, but can be made by a variety of laboratory procedures. For this reason, its spectroscopic signature is very well understood. Structure and spectrum Like sulfur dioxide (and, indeed, most molecules) but unlike sulfur monoxide, disulfur, or dioxygen, the ground state of disulfur monoxide is a singlet. Condensed solid S2O absorbs at (roughly indigo) and (roughly lime). These bands have been assigned to decomposition products S3 and S4. In the ultraviolet, S2O has absorption band systems in the ranges 250–340 nm and 190–240 nm. There are bands at 323.5 and 327.8 nm. The band in the 315–340 nm range is due to the transition. Gaseous disulfur monoxide does not absorb light in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotrope Of Sulfur
The element sulfur exists as many allotropes. In number of allotropes, sulfur is second only to carbon. Greenwood, 652 In addition to the allotropes, each allotrope often exists in polymorphs (different crystal structures of the same covalently bonded Sn molecules) delineated by Greek prefixes (α, β, etc.). Furthermore, because elemental sulfur has been an item of commerce for centuries, its various forms are given traditional names. Early workers identified some forms that have later proved to be single or mixtures of allotropes. Some forms have been named for their appearance, e.g. "mother of pearl sulfur", or alternatively named for a chemist who was pre-eminent in identifying them, e.g. "Muthmann's sulfur I" or "Engel's sulfur". Steudel, 17 The most commonly encountered form of sulfur is the orthorhombic polymorph of , which adopts a puckered ring – or "crown" – structure. Two other polymorphs are known, also with nearly identical molecular structures. Greenwood, 654 I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lazurite
Lazurite, old name '' Azure spar''''Krivovichev V. G.'' Mineralogical glossary. Scientific editor A. G. Bulakh. — St.Petersburg: St.Petersburg Univ. Publ. House. 2009. — 556 p. — ISBN 978-5-288-04863-0. ''(in Russian)'' is a tectosilicate mineral with sulfate, sulfur and chloride with formula . It is a feldspathoid and a member of the sodalite group. Lazurite crystallizes in the isometric system although well‐formed crystals are rare. It is usually massive and forms the bulk of the gemstone lapis lazuli. Mineral Lazurite is a deep‐blue to greenish‐blue. The colour is due to the presence of anions. It has a Mohs hardness of 5.0 to 5.5 and a specific gravity of 2.4. It is translucent with a refractive index of 1.50. It is fusible at 3.5 on Wolfgang Franz von Kobell's fusibility scale, and soluble in HCl. It commonly contains or is associated with grains of pyrite. Lazurite is a product of contact metamorphism of limestone and is typically associated with calcit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 chemical structure, structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetism, diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yves Klein
Yves Klein (; 28 April 1928 – 6 June 1962) was a French artist and an important figure in post-war European art. He was a leading member of the French artistic movement of Nouveau réalisme founded in 1960 by art critic Pierre Restany. Klein was a pioneer in the development of performance art, and is seen as an inspiration to and as a forerunner of Minimalism, minimal art, as well as pop art. He developed and used International Klein Blue. Biography Klein was born in Nice, in the Alpes-Maritimes department of France. His parents, Fred Klein and Marie Raymond, were both painters. His father painted in a loose Post-Impressionism, post-impressionist style, while his mother was a leading figure in Art informel, and held regular soirées with other leading practitioners of this Parisian abstract movement. Klein received no formal training in art, but his parents exposed him to different styles. His father was a figurative style painter, while his mother had an interest in abstract ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Sulfide
Carbonyl sulfide is the chemical compound with the linear formula . It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl double bonded to a sulfur atom. Carbonyl sulfide can be considered to be intermediate between carbon dioxide and carbon disulfide, both of which are valence isoelectronic with it. Occurrence Carbonyl sulfide is the most abundant sulfur compound naturally present in the atmosphere, at , because it is emitted from oceans, volcanoes and deep sea vents. As such, it is a significant compound in the global sulfur cycle. Measurements on the Antarctica ice cores and from air trapped in snow above glaciers ( firn air) have provided a detailed picture of OCS concentrations from 1640 to the present day and allow an understanding of the relative importance of anthropogenic and non-anthropogenic sources of this gas to the atmosphere. Some carbonyl sulfide that is transported into the stratospheric sulfate lay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raman Frequency
Raman spectroscopy () (named after physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. Raman spectroscopy relies upon inelastic scattering of photons, known as Raman scattering. A source of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range is used, although X-rays can also be used. The laser light interacts with molecular vibrations, phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the vibrational modes in the system. Time-resolved spectroscopy and infrared spectroscopy typically yields similar yet complementary information. Typically, a sample is illuminated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lapis Lazuli
Lapis lazuli (; ), or lapis for short, is a deep-blue metamorphic rock used as a semi-precious stone that has been prized since antiquity for its intense color. Originating from the Persian word for the gem, ''lāžward'', lapis lazuli is a rock composed primarily of the minerals lazurite, pyrite and calcite. As early as the 7th millennium BC, lapis lazuli was mined in the Sar-i Sang mines,David Bomford and Ashok Roy, ''A Closer Look- Colour'' (2009), National Gallery Company, London, () in Shortugai, and in other mines in Badakhshan province in modern northeast Afghanistan. Lapis lazuli artifacts, dated to 7570 BC, have been found at Bhirrana, which is the oldest site of Indus Valley civilisation. Lapis was highly valued by the Indus Valley Civilisation (3300–1900 BC). Lapis beads have been found at Neolithic burials in Mehrgarh, the Caucasus, and as far away as Mauritania. It was used in the funeral mask of Tutankhamun (1341–1323 BC). By the end of the Middle A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the structure. For example, , , and are isoelectronic, while and = are not. This definition is sometimes termed valence isoelectronicity. Definitions can sometimes be not as strict, sometimes requiring identity of the total electron count and with it the entire electronic configuration. More usually, definitions are broader, and may extend to allowing different numbers of atoms in the species being compared.A. A. Aradi & T. P. Fehlner, "Isoelectronic Organometallic Molecules", in F. G. A. Stone & Robert West (eds.) ''Advances in Organometallic Chemistry Vol. 30'' (1990), Chapter 5 (at p. 190google books link/ref> The importance of the concept lies in identifying significantly related species, as pairs or series. Isoelectronic species ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |