|
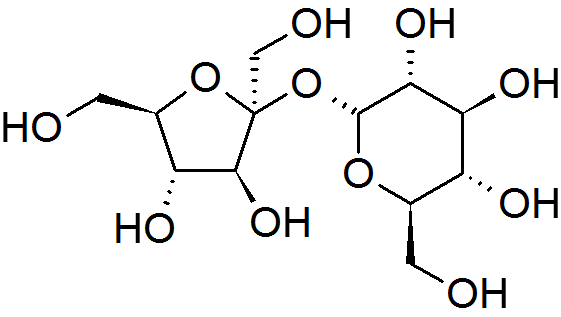
Sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula . For human consumption, sucrose is extracted and refined from either sugarcane or sugar beet. Sugar mills – typically located in tropical regions near where sugarcane is grown – crush the cane and produce raw sugar which is shipped to other factories for refining into pure sucrose. Sugar beet factories are located in temperate climates where the beet is grown, and process the beets directly into refined sugar. The Sugar refinery, sugar-refining process involves washing the raw sugar crystals before dissolving them into a sugar syrup which is filtered and then passed over carbon to remove any residual colour. The sugar syrup is then concentrated by boiling under a vacuum and crystallized as the final purification process to produce crystals of pure sucrose that are clear, odorless, and sweet. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ..., fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is almost pure sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human foo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fructose
Fructose (), or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts most fructose and galactose into glucose for distribution in the bloodstream or deposition into glycogen. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847. The name "fructose" was coined in 1857 by the English chemist William Allen Miller. Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars. Fructose is found in honey, tree and vine fruits, flowers, Berry, berries, and most List of root vegetables, root vegetables. Commercially, fructose is derived from sugar cane, sugar beets, and maize. High-fructose corn syrup is a mixture of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disaccharide
A disaccharide (also called a double sugar or ''biose'') is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose. Disaccharides are one of the four chemical groupings of carbohydrates (monosaccharides, disaccharides, oligosaccharides, and polysaccharides). The most common types of disaccharides—sucrose, lactose, and maltose—have 12 carbon atoms, with the general formula C12H22O11. The differences in these disaccharides are due to atomic arrangements within the molecule. The joining of monosaccharides into a double sugar happens by a condensation reaction, which involves the elimination of a water molecule from the functional groups only. Breaking apart a double sugar into its two monosaccharides is accomplished by hydrolysis with the help of a type of enzyme called a disaccharidase. As building the larger sugar ejects a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fructosyl
Fructosides are glycosides that contain fructose. They are abundant in living organisms, food, and the environment. This makes them a particular interest in pharmacology and food science. C1 of fructose may be bonded to any organic moiety, forming a fructoside. The configuration of the fructoside can be denoted as an α-fructosidase or β-fructosidase depending on whether the organic moiety is bonded below or above the plane of fructose, respectively. The naming of fructoside-related enzymes also follows this nomenclature. Formation and cleavage The formation of fructosides is catalyzed through enzymes called fructosyltransferases. These operate by transferring fructose to other organic moieties. This can be done repeatedly to make polymers or configure addition to diversify the molecule. Fructosyltransferases are most active in forming sucrose-derived fructosides and fructans. The breakdown of fructosides is catalyzed through enzymes called fructosidases. A notable example is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living Organism, organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is -glucose, while its Stereoisomerism, stereoisomer L-glucose, -glucose is produced synthetically in comparatively small amounts and is less biologicall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltose
} Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar. History Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix ' -ose' which is used in names of sugars. Structure and nomenclature Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending on th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Handbook Of Chemistry And Physics
The ''CRC Handbook of Chemistry and Physics'' is a comprehensive one-volume reference resource for science research. First published in 1914, it is currently () in its 105th edition, published in 2024. It is known colloquially among chemists as the "Rubber Bible", as CRC originally stood for "Chemical Rubber Company". As late as the 1962–1963 edition (3604 pages), the ''Handbook'' contained myriad information for every branch of science and engineering. Sections in that edition include: Mathematics, Properties and Physical Constants, Chemical Tables, Properties of Matter, Heat, Hygrometric and Barometric Tables, Sound, Quantities and Units, and Miscellaneous. ''Mathematical Tables from Handbook of Chemistry and Physics'' was originally published as a supplement to the handbook up to the 9th edition (1952); afterwards, the 10th edition (1956) was published separately as '' CRC Standard Mathematical Tables''. Earlier editions included sections such as "Antidotes of Poisons", ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-ose
The suffix ''-ose'' () is used in organic chemistry to form the names of sugars. This Latin suffix means "full of", "abounding in", "given to", or "like". Numerous systems exist to name specific sugars more descriptively. The suffix is also used more generally in English to form adjectives from nouns, with the sense "full of", suffix2 as in "verbose": wordy, full of words. Monosaccharides, the simplest sugars, may be named according to the number of carbon atoms in each molecule of the sugar: pentose is a five-carbon monosaccharide, and hexose is a six-carbon monosaccharide. Aldehyde monosaccharides may be called aldoses; ketone monosaccharides may be called ketoses. Larger sugars such as disaccharides and polysaccharides can be named to reflect their qualities. Lactose, a disaccharide found in milk, gets its name from the Latin word for milk combined with the sugar suffix; its name means "milk sugar". The polysaccharide that makes up plant starch is named amylose, or "starch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marcellin Berthelot
Pierre Eugène Marcellin Berthelot (; 25 October 1827 – 18 March 1907) was a French chemist and Republican politician noted for the ThomsenBerthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substances, providing a large amount of counter-evidence to the theory of Jöns Jakob Berzelius that organic compounds required organisms in their synthesis. Berthelot was convinced that chemical synthesis would revolutionize the food industry by the year 2000, and that synthesized foods would replace farms and pastures. "Why not", he asked, "if it proved cheaper and better to make the same materials than to grow them?" He was considered "one of the most famous chemists in the world." Upon being appointed to the post of Minister of Foreign Affairs for the French government in 1895, he was considered "the most eminent living chemist" in France. In 1901, he was elected as one of the "Forty Immortals" of the Académie française. He gave all his disc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization. Classification Chemistry classifies monomers by type, and two broad classes based on the type of polymer they form. By type: * natural vs synthetic, e.g. glycine vs caprolactam, respectively * polar vs nonpolar, e.g. vinyl acetate vs ethylene, respectively * cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively By type of polymer they form: * those that participate in condensation polymerization * those that participate in addition polymerization Differing stoichiometry causes each class to create its respective form of polymer. : The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucosyl
In organic chemistry, a glycosyl group is a univalent free radical or substituent structure obtained by removing the hydroxyl () group from the hemiacetal () group found in the cyclic form of a monosaccharide and, by extension, of a lower oligosaccharide. Glycosyl groups are exchanged during glycosylation from the glycosyl donor, the electrophile, to the glycosyl acceptor, the nucleophile. The outcome of the glycosylation reaction is largely dependent on the reactivity of each partner. Glycosyl also reacts with inorganic acids, such as phosphoric acid, forming an ester such as glucose 1-phosphate. Examples In cellulose, glycosyl groups link together 1,4-β-D-glucosyl units to form chains of (1,4-β-D-glucosyl)n. Other examples include ribityl in 6,7-Dimethyl-8-ribityllumazine, and glycosylamines. Alternative substituent groups Instead of the hemiacetal hydroxyl group, a ''hydrogen'' atom can be removed to form a substituent, for example the hydrogen from the C3 hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomer
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is a quantum superposition, essentially the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |